Anti-Proliferative Effects of Iridoids from Valeriana fauriei on Cancer Stem Cells
- PMID: 36430685
- PMCID: PMC9698980
- DOI: 10.3390/ijms232214206
Anti-Proliferative Effects of Iridoids from Valeriana fauriei on Cancer Stem Cells
Abstract
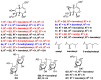
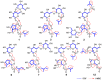
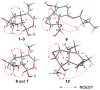
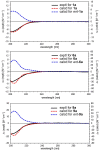
We isolated seven new iridoid glucosides (valerianairidoids I-VII; 1-3, 6, 7, 9, and 12) and six known compounds from the methanol extract of the dried rhizomes and roots of Valeriana fauriei. Chemical and spectroscopic data were used to elucidate the chemical structures of the seven new iridoid glucosides, and their absolute configurations were determined by comparing their electronic circular dichroism (ECD) spectra with those determined experimentally. Aglycones 1a, 6a, and 9a, which were obtained by enzymatic hydrolysis of the isolated iridoid glucosides, exhibited anti-proliferative activities against cancer stem cells (CSCs) established by a sphere-formation assay using human breast cancer (MDA-MB-231) and human astrocytoma (U-251MG) cells. Interestingly, these iridoids selectively showed anti-proliferative activities against CSCs from MDA-MB-231 cells. These results suggest that the iridoids obtained in this study may have potency as a breast cancer treatment and as preventive agent via exterminating CSCs.
Keywords: ECD; Valeriana fauriei; cancer prevention; cancer stem cell; cancer treatment; valerianairidoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wang P.C., Ran X.H., Luo H.R., Hu J.M., Chen R., Ma Q.Y., Dai H.F., Liu Y.Q., Xie M.J., Zhou J., et al. Volvalerelactones A and B, Two New Sesquiterpenoid Lactones with an Unprecedented Skeleton from Valeriana officinalis var. latifolia. Org. Lett. 2011;13:3036–3039. doi: 10.1021/ol200878w. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

