The Lack of STING Impairs the MHC-I Dependent Antigen Presentation and JAK/STAT Signaling in Murine Macrophages
- PMID: 36430709
- PMCID: PMC9697192
- DOI: 10.3390/ijms232214232
The Lack of STING Impairs the MHC-I Dependent Antigen Presentation and JAK/STAT Signaling in Murine Macrophages
Abstract
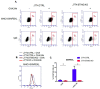
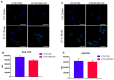
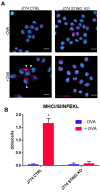
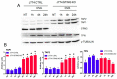
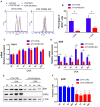
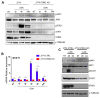
STING is a transmembrane ER resident protein that was initially described as a regulator of innate immune response triggered by viral DNA and later found to be involved in a broader range of immune processes. Here, we assessed its role in the antigen presentation by generating a STING KO macrophage cell line. In the absence of STING, we observed an impaired OVA-derived SIINFEKL peptide presentation together with a decreased level of MHC-I complex on the plasma membrane, likely due to a decreased mRNA expression of β2 m light chain as no relevant alterations of the peptide-loading complex (TAPs) were found. Moreover, JAK-STAT signaling resulted in impaired STING KO cells following OVA and LPS treatments, suggesting a dampened activation of immune response. Our data revealed a new molecular role of STING in immune mechanisms that could elucidate its role in the pathogenesis of autoimmune disorders and cancer.
Keywords: JAK/STAT; STING; antigen presentation; cell biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Srikanth S., Woo J.S., Wu B., El-Sherbiny Y.M., Leung J., Chupradit K., Rice L., Seo G.J., Calmettes G., Ramakrishna C., et al. The ca(2+) sensor stim1 regulates the type i interferon response by retaining the signaling adaptor sting at the endoplasmic reticulum. Nat. Immunol. 2019;20:152–162. doi: 10.1038/s41590-018-0287-8. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

