Radiosensitizing Effect of Trabectedin on Human Soft Tissue Sarcoma Cells
- PMID: 36430780
- PMCID: PMC9698158
- DOI: 10.3390/ijms232214305
Radiosensitizing Effect of Trabectedin on Human Soft Tissue Sarcoma Cells
Abstract
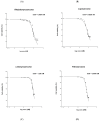
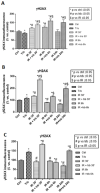
Trabectedin is used for the treatment of advanced soft tissue sarcomas (STSs). In this study, we evaluated if trabectedin could enhance the efficacy of irradiation (IR) by increasing the intrinsic cell radiosensitivity and modulating tumor micro-environment in fibrosarcoma (HS 93.T), leiomyosarcoma (HS5.T), liposarcoma (SW872), and rhabdomyosarcoma (RD) cell lines. A significant reduction in cell surviving fraction (SF) following trabectedin + IR compared to IR alone was observed in liposarcoma and leiomyosarcoma (enhancement ratio at 50%, ER50: 1.45 and 2.35, respectively), whereas an additive effect was shown in rhabdomyosarcoma and fibrosarcoma. Invasive cells' fraction significantly decreased following trabectedin ± IR compared to IR alone. Differences in cell cycle distribution were observed in leiomyosarcoma and rhabdomyosarcoma treated with trabectedin + IR. In all STS lines, trabectedin + IR resulted in a significantly higher number of γ-H2AX (histone H2AX) foci 30 min compared to the control, trabectedin, or IR alone. Expression of ATM, RAD50, Ang-2, VEGF, and PD-L1 was not significantly altered following trabectedin + IR. In conclusion, trabectedin radiosensitizes STS cells by affecting SF (particularly in leiomyosarcoma and liposarcoma), invasiveness, cell cycle distribution, and γ-H2AX foci formation. Conversely, no synergistic effect was observed on DNA damage repair, neoangiogenesis, and immune system.
Keywords: radiation; radiosensitizers; soft tissue sarcoma; trabectedin.
Conflict of interest statement
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; nor in the decision to publish the results.
Figures






References
-
- Casali P.G., Abecassis N., Aro H.T., Bauer S., Biagini R., Bielack S., Bonvalot S., Boukovinas I., Bovee J.V.M.G., Brodowicz T., et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29((Suppl. 4)):iv51–iv67. doi: 10.1093/annonc/mdy096. Erratum in Ann Oncol. 2018, 29 (Suppl. 4), iv268–iv269. - DOI - PubMed
-
- Demetri G.D., von Mehren M., Jones R.L., Hensley M.L., Schuetze S.M., Staddon A., Milhem M., Elias A., Ganjoo K., Tawbi H., et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016;34:786–793. doi: 10.1200/JCO.2015.62.4734. - DOI - PMC - PubMed
-
- Allavena P., Signorelli M., Chieppa M., Erba E., Bianchi G., Marchesi F., Olimpio C.O., Bonardi C., Garbi A., Lissoni A., et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

