Renal Cell Carcinoma as a Metabolic Disease: An Update on Main Pathways, Potential Biomarkers, and Therapeutic Targets
- PMID: 36430837
- PMCID: PMC9698586
- DOI: 10.3390/ijms232214360
Renal Cell Carcinoma as a Metabolic Disease: An Update on Main Pathways, Potential Biomarkers, and Therapeutic Targets
Abstract
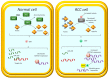
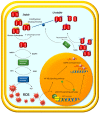
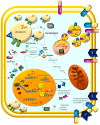
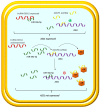
Clear cell renal cell carcinoma (ccRCC) is the most frequent histological kidney cancer subtype. Over the last decade, significant progress has been made in identifying the genetic and metabolic alterations driving ccRCC development. In particular, an integrated approach using transcriptomics, metabolomics, and lipidomics has led to a better understanding of ccRCC as a metabolic disease. The metabolic profiling of this cancer could help define and predict its behavior in terms of aggressiveness, prognosis, and therapeutic responsiveness, and would be an innovative strategy for choosing the optimal therapy for a specific patient. This review article describes the current state-of-the-art in research on ccRCC metabolic pathways and potential therapeutic applications. In addition, the clinical implication of pharmacometabolomic intervention is analyzed, which represents a new field for novel stage-related and patient-tailored strategies according to the specific susceptibility to new classes of drugs.
Keywords: Warburg effect; biomarker; metabolism; metabolomics; renal cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Wei J.-H., Feng Z.-H., Cao Y., Zhao H.-W., Chen Z.-H., Liao B., Wang Q., Han H., Zhang J., Xu Y.-Z., et al. Predictive value of single-nucleotide polymorphism signature for recurrence in localised renal cell carcinoma: A retrospective analysis and multicentre validation study. Lancet Oncol. 2019;20:591–600. doi: 10.1016/S1470-2045(18)30932-X. - DOI - PubMed
-
- Di Lorenzo G., De Placido S., Pagliuca M., Ferro M., Lucarelli G., Rossetti S., Bosso D., Puglia L., Pignataro P., Ascione I., et al. The evolving role of monoclonal antibodies in the treatment of patients with advanced renal cell carcinoma: A systematic review. Exp. Opin. Biol. Ther. 2016;16:1387–1401. doi: 10.1080/14712598.2016.1216964. - DOI - PubMed
-
- Tamma R., Rutigliano M., Lucarelli G., Annese T., Ruggieri S., Cascardi E., Napoli A., Battaglia M., Ribatti D. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol. Oncol. 2019;37:355.e11–355.e19. doi: 10.1016/j.urolonc.2019.01.025. - DOI - PubMed

