Emerging Roles of Type-I Interferons in Neuroinflammation, Neurological Diseases, and Long-Haul COVID
- PMID: 36430870
- PMCID: PMC9696119
- DOI: 10.3390/ijms232214394
Emerging Roles of Type-I Interferons in Neuroinflammation, Neurological Diseases, and Long-Haul COVID
Abstract
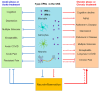
Interferons (IFNs) are pleiotropic cytokines originally identified for their antiviral activity. IFN-α and IFN-β are both type I IFNs that have been used to treat neurological diseases such as multiple sclerosis. Microglia, astrocytes, as well as neurons in the central and peripheral nervous systems, including spinal cord neurons and dorsal root ganglion neurons, express type I IFN receptors (IFNARs). Type I IFNs play an active role in regulating cognition, aging, depression, and neurodegenerative diseases. Notably, by suppressing neuronal activity and synaptic transmission, IFN-α and IFN-β produced potent analgesia. In this article, we discuss the role of type I IFNs in cognition, neurodegenerative diseases, and pain with a focus on neuroinflammation and neuro-glial interactions and their effects on cognition, neurodegenerative diseases, and pain. The role of type I IFNs in long-haul COVID-associated neurological disorders is also discussed. Insights into type I IFN signaling in neurons and non-neuronal cells will improve our treatments of neurological disorders in various disease conditions.
Keywords: IFN-α; IFN-β; astrocytes; long-haul COVID; microglia; neuroinflammation; neurological disease; pain; primary sensory neurons; spinal cord.
Conflict of interest statement
Ru-Rong Ji is a consultant of Boston Scientific and received a research grant from the company. This activity is not related to this study.
Figures
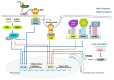
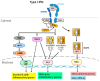
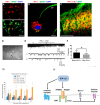
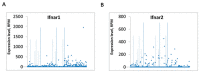

References
-
- Pestka S. The human interferon-alpha species and hybrid proteins. Semin. Oncol. 1997;24:S9-4–S9-17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

