Human Osteoblast-Conditioned Media Can Influence Staphylococcus aureus Biofilm Formation
- PMID: 36430871
- PMCID: PMC9696964
- DOI: 10.3390/ijms232214393
Human Osteoblast-Conditioned Media Can Influence Staphylococcus aureus Biofilm Formation
Abstract
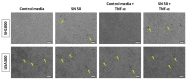
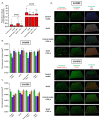
Osteoblasts are bone-forming and highly active cells participating in bone homeostasis. In the case of osteomyelitis and more specifically prosthetic joint infections (PJI) for which Staphylococcus aureus (S. aureus) is mainly involved, the interaction between osteoblasts and S. aureus results in impaired bone homeostasis. If, so far, most of the studies of osteoblasts and S. aureus interactions were focused on osteoblast response following direct interactions with co-culture and/or internalization models, less is known about the effect of osteoblast factors on S. aureus biofilm formation. In the present study, we investigated the effect of human osteoblast culture supernatant on methicillin sensitive S. aureus (MSSA) SH1000 and methicillin resistant S. aureus (MRSA) USA300. Firstly, Saos-2 cell line was incubated with either medium containing TNF-α to mimic the inflammatory periprosthetic environment or with regular medium. Biofilm biomass was slightly increased for both strains in the presence of culture supernatant collected from Saos-2 cells, stimulated or not with TNF-α. In such conditions, SH1000 was able to develop microcolonies, suggesting a rearrangement in biofilm organization. However, the biofilm matrix and regulation of genes dedicated to biofilm formation were not substantially changed. Secondly, culture supernatant obtained from primary osteoblast culture induced varied response from SH1000 strain depending on the different donors tested, whereas USA300 was only slightly affected. This suggested that the sensitivity to bone cell secretions is strain dependent. Our results have shown the impact of osteoblast secretions on bacteria and further identification of involved factors will help to manage PJI.
Keywords: MRSA; MSSA; biofilms; osteoblast secretion; prosthetic joint infection.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Reffuveille F., Josse J., Velard F., Lamret F., Varin-Simon J., Dubus M., Haney E.F., Hancock R., Mongaret C., Gangloff S.C. Bone Environment Influences Irreversible Adhesion of a Methicillin-Susceptible Staphylococcus aureus Strain. Front. Microbiol. 2018;9:2865. doi: 10.3389/fmicb.2018.02865. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

