Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation
- PMID: 36430874
- PMCID: PMC9698208
- DOI: 10.3390/ijms232214396
Emerging Role of NLRP3 Inflammasome and Pyroptosis in Liver Transplantation
Abstract
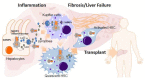
The nucleotide-binding domain leucine-rich repeat-receptor, pyrin domain-containing-3 (NLRP3) inflammasome contributes to the inflammatory response by activating caspase-1, which in turn participates in the maturation of interleukin (IL)-1β and IL-18, which are mainly secreted via pyroptosis. Pyroptosis is a lytic type of cell death that is controlled by caspase-1 processing gasdermin D. The amino-terminal fragment of gasdermin D inserts into the plasma membrane, creating stable pores and enabling the release of several proinflammatory factors. The activation of NLRP3 inflammasome and pyroptosis has been involved in the progression of liver fibrosis and its end-stage cirrhosis, which is among the main etiologies for liver transplantation (LT). Moreover, the NLRP3 inflammasome is involved in ischemia-reperfusion injury and early inflammation and rejection after LT. In this review, we summarize the recent literature addressing the role of the NLRP3 inflammasome and pyroptosis in all stages involved in LT and argue the potential targeting of this pathway as a future therapeutic strategy to improve LT outcomes. Likewise, we also discuss the impact of graft quality influenced by donation after circulatory death and the expected role of machine perfusion technology to modify the injury response related to inflammasome activation.
Keywords: NLRP3 inflammasome; graft rejection; ischemia-reperfusion injury; liver transplantation; pyroptosis.
Conflict of interest statement
P.P., A.B.-M. and S.C. are co-founders of Viva in vitro diagnostics SL but declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The other authors declare no competing interests.
Figures


References
-
- Baroja-Mazo A., Martin-Sanchez F., Gomez A.I., Martinez C.M., Amores-Iniesta J., Compan V., Barbera-Cremades M., Yague J., Ruiz-Ortiz E., Anton J., et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

