Recent Advances in Nanomaterials for Asthma Treatment
- PMID: 36430906
- PMCID: PMC9696023
- DOI: 10.3390/ijms232214427
Recent Advances in Nanomaterials for Asthma Treatment
Abstract
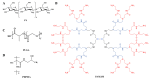
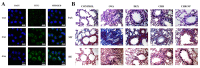
Asthma is a chronic airway inflammatory disease with complex mechanisms, and these patients often encounter difficulties in their treatment course due to the heterogeneity of the disease. Currently, clinical treatments for asthma are mainly based on glucocorticoid-based combination drug therapy; however, glucocorticoid resistance and multiple side effects, as well as the occurrence of poor drug delivery, require the development of more promising treatments. Nanotechnology is an emerging technology that has been extensively researched in the medical field. Several studies have shown that drug delivery systems could significantly improve the targeting, reduce toxicity and improve the bioavailability of drugs. The use of multiple nanoparticle delivery strategies could improve the therapeutic efficacy of drugs compared to traditional delivery methods. Herein, the authors presented the mechanisms of asthma development and current therapeutic methods. Furthermore, the design and synthesis of different types of nanomaterials and micromaterials for asthma therapy are reviewed, including polymetric nanomaterials, solid lipid nanomaterials, cell membranes-based nanomaterials, and metal nanomaterials. Finally, the challenges and future perspectives of these nanomaterials are discussed to provide guidance for further research directions and hopefully promote the clinical application of nanotherapeutics in asthma treatment.
Keywords: asthma; biomedical polymers; drug delivery; nanomaterials; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- NO. 52073278/National Natural Science Foundation of China
- NO. 2021JC036/Health Commission of Jilin Province
- (NO.20200601011JC)/Key Laboratory of Precision Infectious Diseases of Jilin Province
- NO. 3D5200117426/Jilin Provincial Key Laboratory of Pathogen Biology
- NO. 3J1222527426/Jilin Provincial Development and Reform Commission Common Disease Precision Prevention and Control Engineering Laboratory
LinkOut - more resources
Full Text Sources
Medical

