Loss of Runx1 Induces Granulosa Cell Defects and Development of Ovarian Tumors in the Mouse
- PMID: 36430923
- PMCID: PMC9697285
- DOI: 10.3390/ijms232214442
Loss of Runx1 Induces Granulosa Cell Defects and Development of Ovarian Tumors in the Mouse
Abstract
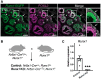
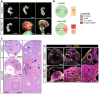
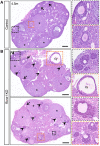
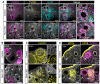
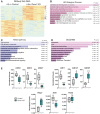
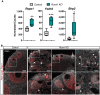
Genetic alterations of the RUNX1 gene are associated with a variety of malignancies, including female-related cancers. The role of RUNX1 as either a tumor suppressor gene or an oncogene is tissue-dependent and varies based on the cancer type. Both the amplification and deletion of the RUNX1 gene have been associated with ovarian cancer in humans. In this study, we investigated the effects of Runx1 loss on ovarian pathogenesis in mice. A conditional loss of Runx1 in the somatic cells of the ovary led to an increased prevalence of ovarian tumors in aged mice. By the age of 15 months, 27% of Runx1 knockout (KO) females developed ovarian tumors that presented characteristics of granulosa cell tumors. While ovaries from young adult mice did not display tumors, they all contained abnormal follicle-like lesions. The granulosa cells composing these follicle-like lesions were quiescent, displayed defects in differentiation and were organized in a rosette-like pattern. The RNA-sequencing analysis further revealed differentially expressed genes in Runx1 KO ovaries, including genes involved in metaplasia, ovarian cancer, epithelial cell development, tight junctions, cell-cell adhesion, and the Wnt/beta-catenin pathway. Together, this study showed that Runx1 is required for normal granulosa cell differentiation and prevention of ovarian tumor development in mice.
Keywords: RUNX1; cancer; granulosa cells; ovary; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
RUNX1 maintains the identity of the fetal ovary through an interplay with FOXL2.Nat Commun. 2019 Nov 11;10(1):5116. doi: 10.1038/s41467-019-13060-1. Nat Commun. 2019. PMID: 31712577 Free PMC article.
-
Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development.Cancer Res. 2005 Oct 15;65(20):9206-15. doi: 10.1158/0008-5472.CAN-05-1024. Cancer Res. 2005. PMID: 16230381
-
Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model.Cancer Res. 2006 Feb 15;66(4):1964-73. doi: 10.1158/0008-5472.CAN-05-3493. Cancer Res. 2006. PMID: 16488995
-
The enigmatic role of RUNX1 in female-related cancers - current knowledge & future perspectives.FEBS J. 2017 Aug;284(15):2345-2362. doi: 10.1111/febs.14059. Epub 2017 Apr 10. FEBS J. 2017. PMID: 28304148 Review.
-
Transgenic mice expressing inhibin α-subunit promoter (inhα)/Simian Virus 40 T-antigen (Tag) transgene as a model for the therapy of granulosa cell-derived ovarian cancer.Reprod Biol. 2014 Mar;14(1):25-31. doi: 10.1016/j.repbio.2013.11.005. Epub 2013 Dec 11. Reprod Biol. 2014. PMID: 24607252 Review.
Cited by
-
RUNX1-MUC13 Interaction Activates Wnt/β-Catenin Signaling Implications for Colorectal Cancer Metastasis.Int J Biol Sci. 2024 Sep 16;20(12):4999-5026. doi: 10.7150/ijbs.98396. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309442 Free PMC article.
-
RUN(X) out of blood: emerging RUNX1 functions beyond hematopoiesis and links to Down syndrome.Hum Genomics. 2023 Sep 5;17(1):83. doi: 10.1186/s40246-023-00531-2. Hum Genomics. 2023. PMID: 37670378 Free PMC article. Review.
-
Gonadal sex reversal at single-cell resolution in Znrf3-deficient mice.Development. 2024 Dec 1;151(23):dev202707. doi: 10.1242/dev.202707. Epub 2024 Dec 4. Development. 2024. PMID: 39629665 Free PMC article.
References
-
- De Braekeleer E., Ferec C., De Braekeleer M. RUNX1 translocations in malignant hemopathies. Anticancer Res. 2009;29:1031–1037. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

