Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats
- PMID: 36430984
- PMCID: PMC9697363
- DOI: 10.3390/life12111850
Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats
Abstract
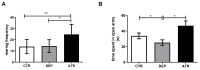
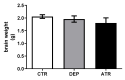
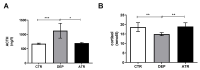
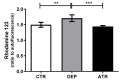
Atranorin (ATR) is one of lichens' many known secondary metabolites. Most current studies have investigated the various effects of ATR in vitro and only sporadically in vivo. The latest data indicate that ATR may have anxiolytic/antidepressive effects. This study aimed to analyze the potential of ATR in a depression-like state in male Wistar rats. Pregnant females were stressed by restricting their mobility in the final week of pregnancy three times a day for 45 min each, for three following days. After birth, progeny aged 60 days was stressed repeatedly. The male progeny was divided into three groups as follows: CTR group as a healthy control (n = 10), DEP group as a progeny of restricted mothers (n = 10), and ATR group as a progeny of restricted mothers, treated daily for one month with ATR (n = 10; 10 mg/kg of body weight, p.o.). Our results show that ATR acts as an antioxidant and markedly changes animal behavior. Concomitantly, hippocampal neurogenesis increases in the hilus and subgranular zone, together with the number of NeuN mature neurons in the hilus and CA1 regions. Our results indicate a potential antidepressant/anxiolytic effect of ATR. However, further studies in this area are needed.
Keywords: Wistar rats; anxiety; atranorin; depression; hippocampus; neurogenesis; reactive oxygen species; stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Santomauro D.F., Herrera A.M.M., Shadid J., Zheng P., Ashbaugh C., Pigott D.M., Abbafati C., Adolph C., Amlag J.O., Aravkin A.Y. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. - DOI - PMC - PubMed
-
- Lund C., Brooke-Sumner C., Baingana F., Baron E.C., Breuer E., Chandra P., Haushofer J., Herrman H., Jordans M., Kieling C. Social determinants of mental disorders and the Sustainable Development Goals: A systematic review of reviews. Lancet Psychiatry. 2018;5:357–369. doi: 10.1016/S2215-0366(18)30060-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

