Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation-Results from a Large Animal Model
- PMID: 36430994
- PMCID: PMC9694613
- DOI: 10.3390/life12111859
Concomitant Intra-Aortic Balloon Pumping Significantly Reduces Left Ventricular Pressure during Central Veno-Arterial Extracorporeal Membrane Oxygenation-Results from a Large Animal Model
Abstract
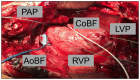
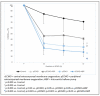
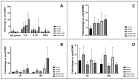
(1) Introduction: Simultaneous ECMO and IABP therapy is frequently used. Haemodynamic changes responsible for the success of the concomitant mechanical circulatory support system approach are rarely investigated. In a large-animal model, we analysed haemodynamic parameters before and during ECMO therapy, comparing central and peripheral ECMO circulation with and without simultaneous IABP support. (2) Methods: Thirty-three female pigs were divided into five groups: (1) SHAM, (2) (peripheral)ECMO(-)IABP, (3) (p)ECMO(+)IABP, (4) (central)ECMO(-)IABP, and (5) (c)ECMO(+)IABP. Pigs were cannulated in accordance with the group and supported with ECMO (±IABP) for 10 h. Systemic haemodynamics, cardiac index (CI), and coronary and carotid artery blood flow were determined before, directly after, and at five and ten hours on extracorporeal support. Systemic inflammation (IL-6; IL-10; TNFα; IFNγ), immune response (NETs; cf-DNA), and endothelial injury (ET-1) were also measured. (3) Results: IABP support during antegrade ECMO circulation led to a significant reduction of left ventricular pressure in comparison to retrograde flow in (p)ECMO(-)IABP and (p)ECMO(+)IABP. Blood flow in the left anterior coronary and carotid artery was not affected by extracorporeal circulation. (4) Conclusions: Concomitant central ECMO and IABP therapy leads to significant reduction of intracavitary cardiac pressure, reduces cardiac work, and might therefore contribute to improved recovery in ECMO patients.
Keywords: ECMO; IABP; cardiogenic shock; left ventricular pressure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Acharya D., Loyaga-Rendon R.Y., Tallaj J.A., Pamboukian S.V., Sasse M.F. Circulatory support for shock complicating myocardial infarction. J. Invasive Cardiol. 2014;26:E109–E114. - PubMed
-
- Mebazaa A., Pitsis A.A., Rudiger A., Toller W., Longrois D., Ricksten S.-E., Bobek I., De Hert S., Wieselthaler G., Schirmer U., et al. Clinical review: Practical recom-mendations on the management of perioperative heart failure in cardiac surgery. Crit. Care. 2010;14:201. doi: 10.1186/cc8153. - DOI - PMC - PubMed
-
- Russo J.J., Aleksova N., Pitcher I., Couture E., Parlow S., Faraz M., Visintini S., Simard T., Di Santo P., Mathew R., et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients with Cardiogenic Shock. J. Am. Coll. Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085. - DOI - PubMed
-
- Vallabhajosyula S., O’Horo J.C., Antharam P., Ananthaneni S., Vallabhajosyula S., Stulak J.M., Dunlay S.M., Holmes D.R., Jr., Barsness G.W. Venoarterial Extracorporeal Membrane Oxygenation with Concomitant Impella Versus Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock. ASAIO J. 2020;66:497–503. doi: 10.1097/MAT.0000000000001039. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

