Altered Serum Uric Acid Levels in Kidney Disorders
- PMID: 36431026
- PMCID: PMC9692609
- DOI: 10.3390/life12111891
Altered Serum Uric Acid Levels in Kidney Disorders
Abstract
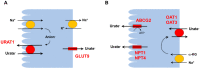
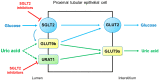
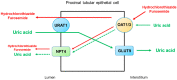
Serum uric acid levels are altered by kidney disorders because the kidneys play a dominant role in uric acid excretion. Here, major kidney disorders which accompany hyperuricemia or hypouricemia, including their pathophysiology, are discussed. Chronic kidney disease (CKD) and hyperuricemia are frequently associated, but recent clinical trials have not supported the pathogenic roles of hyperuricemia in CKD incidence and progression. Diabetes mellitus (DM) is often associated with hyperuricemia, and hyperuricemia may be associated with an increased risk of diabetic kidney disease in patients with type 2 DM. Sodium-glucose cotransporter 2 inhibitors have a uricosuric effect and can relieve hyperuricemia in DM. Autosomal dominant tubulointerstitial kidney disease (ADTKD) is an important hereditary kidney disease, mainly caused by mutations of uromodulin (UMOD) or mucin-1 (MUC-1). Hyperuricemia and gout are the major clinical manifestations of ADTKD-UMOD and ADTKD-MUC1. Renal hypouricemia is caused by URAT1 or GLUT9 loss-of-function mutations and renders patients susceptible to exercise-induced acute kidney injury, probably because of excessive urinary uric acid excretion. Hypouricemia derived from renal uric acid wasting is a component of Fanconi syndrome, which can be hereditary or acquired. During treatment for human immunodeficiency virus, hepatitis B or cytomegalovirus, tenofovir, adefovir, and cidofovir may cause drug-induced renal Fanconi syndrome. In coronavirus disease 2019, hypouricemia due to proximal tubular injury is related to disease severity, including respiratory failure. Finally, serum uric acid and the fractional excretion of uric acid are indicative of plasma volume status; hyperuricemia caused by the enhanced uric acid reabsorption can be induced by volume depletion, and hypouricemia caused by an increased fractional excretion of uric acid is the characteristic finding in syndromes of inappropriate anti-diuresis, cerebral/renal salt wasting, and thiazide-induced hyponatremia. Molecular mechanisms by which uric acid transport is dysregulated in volume or water balance disorders need to be investigated.
Keywords: COVID-19; Fanconi syndrome; autosomal dominant tubulointerstitial kidney disease; chronic kidney disease; hyperuricemia; hyponatremia; hypouricemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yanai H., Adachi H., Hakoshima M., Katsuyama H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 2021;22:9221. doi: 10.3390/ijms22179221. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

