The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study
- PMID: 36431332
- PMCID: PMC9692274
- DOI: 10.3390/jcm11226855
The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study
Abstract
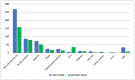
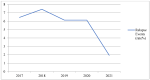
In the current COVID-19 pandemic, patients diagnosed with multiple sclerosis (MS) are considered to be one of the highest priority categories, being recognized as extremely vulnerable people. For this reason, mRNA-based COVID-19 vaccines are strongly recommended for these patients. Despite encouraging results on the efficacy and safety profile of mRNA-based COVID-19 vaccines, to date, in frail populations, including patients diagnosed with MS, this information is rather limited. We carried out a retrospective observational study with the aim to evaluate the safety profile of mRNA-based COVID-19 vaccines by retrieving real-life data of MS patients who were treated and vaccinated at the Multiple Sclerosis Center of the Hospital A.O.R.N. A. Cardarelli. Three-hundred and ten medical records of MS patients who received the first dose of the mRNA-based COVID-19 vaccine were retrieved (63% female; mean age: 45.9 years). Of these patients, 288 also received the second dose. All patients received the Pfizer-BioNTech vaccine. Relapsing-Remitting Multiple Sclerosis (RRSM) was the most common form of MS. The Expanded Disability Status Scale (EDSS) values were <3.0 in 70% of patients. The majority of patients received a Disease Modifying Therapy (DMT) during the study period, mainly interferon beta 1-a, dimethyl fumarate, and natalizumab and fingolimod. Overall, 913 AEFIs were identified, of which 539 were after the first dose of the vaccine and 374 after the second dose. The majority of these AEFIs were classified as short-term since they occurred within the first 72 h. The most common identified adverse events were pain at injection site, flu-like symptoms, and headache. Fever was reported more frequently after the second dose than after the first dose. SARS-CoV-2 infection occurred in 3 patients after the first dose. Using historical data of previous years (2017−2020), the relapses’ rate during 2021 was found to be lower. Lastly, the results of the multivariable analysis that assessed factors associated with the occurrence of AEFIs revealed a statistical significance for age, sex, and therapy with ocrelizumab (p < 0.05). In conclusion, our results indicated that Pfizer-BioNTech vaccine was safe for MS patients, being associated with AEFIs already detected in the general population. Larger observational studies with longer follow-up and epidemiological studies are strongly needed.
Keywords: AEFI; COVID-19; mRNA-based vaccine; multiple sclerosis; observational study; safety.
Conflict of interest statement
The authors declare no conflict of interest. G.T.M. received personal compensation from Serono, Biogen, Novartis, Roche, and TEVA for public speaking and advisory boards. C.S., A.M., V.M., E.P., G.G., M.L.A., S.C., A.B., O.M., D.D.G.C., A.R.Z., A.F., M.M. (Marida Massa), M.M. (Massimo Majolo), E.R., R.S., G.R., G.L., V.A. and A.C. have no disclosures.
Figures
References
-
- European Medicine Agency Comirnaty (COVID-19 mRNA Vaccine [Nucleoside Modified]). An Overview of Comirnaty and Why It Is Authorised in the EU. [(accessed on 16 September 2021)]; Available online: https://www.ema.europa.eu/en/documents/overview/comirnaty-epar-medicine-....
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous