Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study
- PMID: 36431337
- PMCID: PMC9693598
- DOI: 10.3390/jcm11226860
Effectiveness and Safety of Ultrasound-Guided Local Paricalcitol Injection in Treating Secondary Hyperparathyroidism in ESRD: A Retrospective Study
Abstract
Purpose: To compare the safety and efficacy of percutaneous paricalcitol injection with intravenously administered paricalcitol in treating parathyroid hyperplasia in patients with secondary hyperparathyroidism (SHPT).
Methods: This study was approved by the Ethics Committee of our institution. We retrospectively collected data on patients who received percutaneous paricalcitol injection (24 patients) and intravenously administered paricalcitol (22 patients) based on their intact parathyroid hormone (iPTH) level. Serum iPTH, calcium, phosphorus, and the volume of the parathyroid gland were measured at several indicated time points after treatment, and adverse events associated with the two treatments were evaluated.
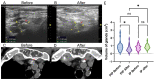
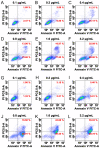
Results: After 6 months of follow-up, we found that patients from the percutaneous injection group had significantly decreased levels of iPTH (from 1887.81 ± 726.81 pg/mL to 631.06 ± 393.06 pg/mL), phosphate (from 1.94 ± 0.36 mmol/L to 1.71 ± 0.34 mmol/L), and volume of the parathyroid gland (from 0.87 ± 0.50 cm3 to 0.60 ± 0.36 cm3), with relief from ostealgia within 48-72 h. In the intravenously administered group, the levels of iPTH decreased from 686.87 ± 260.44 pg/mL to 388.47 ± 167.36 pg/mL; while there was no significant change in phosphate levels, the volume of the parathyroid gland and ostealgia relief were observed at the end of follow-up. The serum calcium level did not significantly change, and no severe complications were observed in both groups. In vitro fluorescence-activated single cell sorting (FACS) analysis indicated that paricalcitol induced parathyroid cell apoptosis in a dose-dependent manner.
Conclusions: Percutaneous paricalcitol injection is a selective treatment for SHPT in ESRD.
Keywords: end stage renal disease (ESRD); intravenously paricalcitol (IP); local paricalcitol injection (LPI); secondary hyperparathyroidism (SHPT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Treatment of secondary hyperparathyroidism with paricalcitol in patients with end-stage renal disease undergoing hemodialysis in Turkey: an observational study.Int Urol Nephrol. 2019 Jul;51(7):1261-1270. doi: 10.1007/s11255-019-02175-5. Epub 2019 Jun 3. Int Urol Nephrol. 2019. PMID: 31161518
-
Paricalcitol in hemodialysis patients with secondary hyperparathyroidism and its potential benefits.World J Clin Cases. 2021 Nov 26;9(33):10172-10179. doi: 10.12998/wjcc.v9.i33.10172. World J Clin Cases. 2021. PMID: 34904087 Free PMC article. Clinical Trial.
-
19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis.J Am Soc Nephrol. 1998 Aug;9(8):1427-32. doi: 10.1681/ASN.V981427. J Am Soc Nephrol. 1998. PMID: 9697664 Clinical Trial.
-
A systematic review of the pharmacotherapy of secondary hyperparathyroidism (SHPT) in grades 3-5 Chronic Kidney Disease (CKD).Eur Rev Med Pharmacol Sci. 2022 Jan;26(1):232-239. doi: 10.26355/eurrev_202201_27773. Eur Rev Med Pharmacol Sci. 2022. PMID: 35049000
-
Paricalcitol, a new agent for the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis.Clin Ther. 1999 Mar;21(3):432-41. doi: 10.1016/S0149-2918(00)88299-5. Clin Ther. 1999. PMID: 10321413 Review.
Cited by
-
Percutaneous ethanol and calcitriol injection therapy for hyperparathyroidism - a single-centre experience.Front Endocrinol (Lausanne). 2025 Jun 4;16:1562493. doi: 10.3389/fendo.2025.1562493. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40535335 Free PMC article.
References
-
- Tentori F., Wang M., Bieber B.A., Karaboyas A., Li Y., Jacobson S.H., Andreucci V.E., Fukagawa M., Frimat L., Mendelssohn D.C., et al. Recent Changes in Therapeutic Approaches and Association with Outcomes among Patients with Secondary Hyperparathyroidism on Chronic Hemodialysis: The DOPPS Study. Clin. J. Am. Soc. Nephrol. 2015;10:98–109. doi: 10.2215/CJN.12941213. - DOI - PMC - PubMed
-
- Ogata H., Fukagawa M., Hirakata H., Kagimura T., Fukushima M., Akizawa T., Suzuki M., Nishizawa Y., Yamazaki C., Tanaka S., et al. Effect of Treating Hyperphosphatemia with Lanthanum Carbonate vs Calcium Carbonate on Cardiovas-cular Events in Patients with Chronic Kidney Disease Undergoing Hemodialysis: The LANDMARK Randomized Clinical Trial. JAMA. 2021;325:1946–1954. doi: 10.1001/jama.2021.4807. - DOI - PMC - PubMed
-
- Parfrey P.S., Chertow G.M., Block G.A., Correa-Rotter R., Drüeke T.B., Floege J., Herzog C.A., London G.M., Mahaffey K.W., Moe S.M., et al. The Clinical Course of Treated Hyperparathyroidism Among Patients Receiving Hemodialysis and the Effect of Cinacalcet: The EVOLVE Trial. J. Clin. Endocrinol. Metab. 2013;98:4834–4844. doi: 10.1210/jc.2013-2975. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

