Sublingual Sufentanil Tablet System (SSTS-Zalviso®) for Postoperative Analgesia after Orthopedic Surgery: A Retrospective Study
- PMID: 36431339
- PMCID: PMC9698499
- DOI: 10.3390/jcm11226864
Sublingual Sufentanil Tablet System (SSTS-Zalviso®) for Postoperative Analgesia after Orthopedic Surgery: A Retrospective Study
Abstract
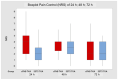
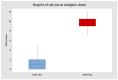
Background: The aim of this study is to compare sublingual sufentanil and the administration device for its delivery (SSST-Zalviso®) with the traditional strategies used for the control of postoperative pain to establish if there is an actual benefit for the patient and healthcare personnel. Materials and Methods: A retrospective study was conducted to compare the efficacy of SSTS in the management of postoperative pain after orthopedic surgery between October 2018 and June 2020. We analyzed 50 patients who underwent a total knee arthroplasty (TKA). The control group consisted of 21 patients who underwent TKA and during the hospitalized recovery received a continuous femoral nerve block (cFNB). The statistical study was conducted with a level of significance p = 0.05 using “U” test, Mann−Whitney, to verify if patients had a better control of pain and fewer calls for rescue analgesia. Results: Patients involved in the study showed a significant reduction in pain intensity with the use of SSTS in the 24 h following surgery (p = 0.0568), also a drastic drop of the calls for rescue analgesia (p < 0.0001) reduces the number of calls for its control. Conclusions: This study demonstrates how SSTS might reduce pain intensity in the first 24 h after surgery and reduce the number of calls for its control, indicating better analgesic coverage and implying reduced interventions from healthcare personnel. This could allow a redistribution of resources and a reduction in the use of analgesic drugs in wards where the SSTS is used.
Keywords: continuous femoral nerve block; postoperative pain; retrospective study; sublingual sufentanil tablet system (SSTS–Zalviso®); total knee arthroplasty.
Conflict of interest statement
Ruggieri reports he is a consultant and designer for Stryker and Exactech (no relationship with the present work). The other authors declare that there are no relationships/conditions/circumstances that present a potential conflict of interest with the present manuscript.
Figures



Similar articles
-
Sublingual sufentanil tablet system Zalviso® for postoperative analgesia after knee replacement in fast track surgery: a pilot observational study.J Exp Orthop. 2018 Mar 20;5(1):8. doi: 10.1186/s40634-018-0123-y. J Exp Orthop. 2018. PMID: 29557999 Free PMC article.
-
Sufentanil sublingual tablet system versus oral oxycodone for management of postoperative pain in enhanced recovery after surgery pathway for total knee arthroplasty: a randomized controlled study.J Exp Orthop. 2020 Nov 20;7(1):92. doi: 10.1186/s40634-020-00306-x. J Exp Orthop. 2020. PMID: 33216238 Free PMC article.
-
Real-world use of the sufentanil sublingual tablet system for patient-controlled management of acute postoperative pain: a prospective noninterventional study.Curr Med Res Opin. 2020 Feb;36(2):277-284. doi: 10.1080/03007995.2019.1681133. Epub 2019 Oct 30. Curr Med Res Opin. 2020. PMID: 31612723
-
Sublingual sufentanil (Zalviso) patient-controlled analgesia after total knee arthroplasty: a retrospective comparison with oxycodone with or without dexamethasone.J Pain Res. 2018 Dec 14;11:3205-3210. doi: 10.2147/JPR.S185197. eCollection 2018. J Pain Res. 2018. PMID: 30588072 Free PMC article. Review.
-
Sublingual Sufentanil: A Review in Acute Postoperative Pain.Drugs. 2016 Apr;76(6):719-29. doi: 10.1007/s40265-016-0571-6. Drugs. 2016. PMID: 27067596 Review.
Cited by
-
Sublingual sufentanil after orthopaedic and abdominal surgery: long-term outcome and safety.Perioper Med (Lond). 2025 Feb 28;14(1):23. doi: 10.1186/s13741-025-00506-y. Perioper Med (Lond). 2025. PMID: 40022238 Free PMC article.
References
-
- Berninger M.T., Friederichs J., Leidinger W., Augat P., Bühren V., Fulghum C., Reng W. Effect of local infiltration analgesia, peripheral nerve blocks, general and spinal anesthesia on early functional recovery and pain control in total knee arthroplasty. BMC Musculoskelet. Disord. 2018;19:232. doi: 10.1186/s12891-018-2154-z. - DOI - PMC - PubMed
-
- van Veen D.E., Verhelst C.C., van Dellen R.T., Koopman J. Sublingual sufentanil (Zalviso) patient-controlled analgesia after total knee arthroplasty: A retrospective comparison with oxycodone with or without dexamethasone. J. Pain Res. 2018;11:3205–3210. doi: 10.2147/JPR.S185197. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

