Effect of Cu Addition on the Corrosion and Antifouling Properties of PEO Coated Zinc-Aluminized Steel
- PMID: 36431381
- PMCID: PMC9692627
- DOI: 10.3390/ma15227895
Effect of Cu Addition on the Corrosion and Antifouling Properties of PEO Coated Zinc-Aluminized Steel
Abstract
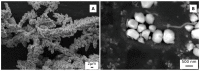
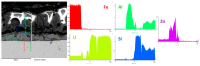
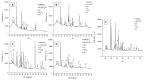
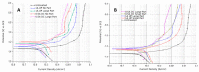
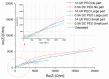
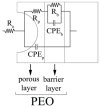
In the present work, Plasma Electrolytic Oxidation (PEO) coatings were produced on zinc-aluminized carbon steels (Galvalume commercial treatment). In addition, copper particles of various sizes were introduced into the coating in order to produce samples with antifouling properties. The particles were successfully embedded into the coating. A higher number of embedded particles was observed when these are in sub-micrometric size and obtained in pulsed current. The presence of particles produces significant antifouling properties on the sample's surfaces during the first 20 days of immersion. The presence of the particles reduces the corrosion resistance in comparison to the samples PEO coated without the particles; however, the corrosion resistance remain higher than the one of the untreated sample.
Keywords: Galvalume; Plasma Electrolytic Oxidation; anti-corrosion coatings; antifouling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Wang Y.L., Wang M., Zhou M., Li B.J., Amoako G., Jiang Z.H. Microstructure characterisation of alumina coating on steel by PEO. Surf. Eng. 2013;29:271–275. doi: 10.1179/1743294412Y.0000000084. - DOI
-
- Attarzadeh N., Molaei M., Babaei K., Fattah-alhosseini A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surf. Interfaces. 2021;23:100997. doi: 10.1016/j.surfin.2021.100997. - DOI
-
- Yerokhin A.L., Snizhko L.O., Gurevina N.L., Leyland A., Pilkington A., Matthews A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminium alloy. Surf. Coat. Technol. 2004;177–178:779–783. doi: 10.1016/j.surfcoat.2003.06.020. - DOI
-
- Hryniewicz T. Plasma electrolytic oxidation of metals and alloys. Metals. 2018;8:1058. doi: 10.3390/met8121058. - DOI
-
- Egorkin V.S., Gnedenkov S.V., Sinebryukhov S.L., Vyaliy I.E., Gnedenkov A.S., Chizhikov R.G. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coat. Technol. 2018;334:29–42. doi: 10.1016/j.surfcoat.2017.11.025. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

