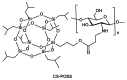
Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering
- PMID: 36431692
- PMCID: PMC9692765
- DOI: 10.3390/ma15228208
Chitosan/POSS Hybrid Hydrogels for Bone Tissue Engineering
Abstract
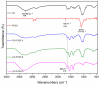
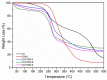
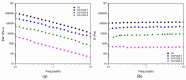
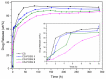
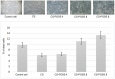
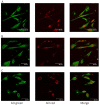
Hybrid hydrogels composed of chitosan (CS) have shown great potential in bone tissue engineering and regeneration. The introduction of polyhedral oligomeric silsesquioxanes (POSS) in the biopolymeric matrix has been demonstrated to improve the rheological and biological properties of the hybrid composites. In this work, we have integrated the favourable features of chitosan (CS) and POSS nanoparticles to design new nanocomposites for bone tissue regeneration, focusing our attention on the effect of POSS concentration within the CS matrix (0.5, 1, and 1.5 equivalents in weight of POSS with respect to CS) on the chemical, physical, rheological, and in vitro biological properties of the final composites. The drug release ability of the synthesized hydrogel scaffolds were also investigated using, as the model drug, ketoprofen, that was included in the scaffold during the gelling procedure, showing a more controlled release for the hybrids with respect to CS (86-91% of drug released after two weeks). The results of the in vitro biological tests performed on human fetal osteoblastic cells (hFOB 1.19) culture demonstrated the great biocompatibility of the hybrid materials. The hybrids, at the different POSS concentrations, showed values of cell mortality superimposable with control cells (11.1 vs. 9.8%), thus revealing the CS/POSS hydrogels as possible candidates for bone tissue engineering applications.
Keywords: biomaterials; biopolymers; hybrid materials; hydrogels; polyhedral oligomeric silsesquioxanes; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wu A.M., Bisignano C., James S.L., Abady G.G. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2:e580–e592. doi: 10.1016/S2666-7568(21)00172-0. - DOI - PMC - PubMed
-
- Koons G.L., Diba M., Mikos A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020;5:584–603. doi: 10.1038/s41578-020-0204-2. - DOI
LinkOut - more resources
Full Text Sources

