Catalytic Enantioselective Synthesis of N-C Axially Chiral N-(2,6-Disubstituted-phenyl)sulfonamides through Chiral Pd-Catalyzed N-Allylation
- PMID: 36431920
- PMCID: PMC9698006
- DOI: 10.3390/molecules27227819
Catalytic Enantioselective Synthesis of N-C Axially Chiral N-(2,6-Disubstituted-phenyl)sulfonamides through Chiral Pd-Catalyzed N-Allylation
Abstract
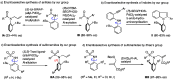
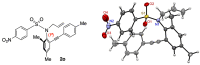
Recently, catalytic enantioselective syntheses of N-C axially chiral compounds have been reported by many groups. Most N-C axially chiral compounds prepared through a catalytic asymmetric reaction possess carboxamide or nitrogen-containing aromatic heterocycle skeletons. On the other hand, although N-C axially chiral sulfonamide derivatives are known, their catalytic enantioselective synthesis is relatively underexplored. We found that the reaction (Tsuji-Trost allylation) of allyl acetate with secondary sulfonamides bearing a 2-arylethynyl-6-methylphenyl group on the nitrogen atom proceeds with good enantioselectivity (up to 92% ee) in the presence of (S,S)-Trost ligand-(allyl-PdCl)2 catalyst, affording rotationally stable N-C axially chiral N-allylated sulfonamides. Furthermore, the absolute stereochemistry of the major enantiomer was determined by X-ray single crystal structural analysis and the origin of the enantioselectivity was considered.
Keywords: N-allylation; asymmetric catalyst; atropisomers; axial chirality; palladium; sulfonamides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Alkorta I., Elguero J., Roussel C., Vanthuyne N., Piras P. Atropisomerism and Axial Chirality in Heteroaromatic Compounds. Adv. Heterocycl. Chem. 2012;105:1–188. doi: 10.1016/B978-0-12-396530-1.00001-2. - DOI
-
- Takahashi I., Suzuki Y., Kitagawa O. Asymmetric Synthesis of Atropisomeric Compounds with an N–C Chiral Axis. Org. Prep. Proced. Int. 2014;46:1–23. doi: 10.1080/00304948.2014.866467. - DOI
-
- Wu Y.-J., Liao G., Shi B.-F. Stereoselective construction of atropisomers featuring a C–N chiral axis. Green Synth. Catal. 2022;3:117–136. doi: 10.1016/j.gresc.2021.12.005. - DOI
-
- Sweet J.S., Knipe P.C. Catalytic Enantioselective Synthesis of C-N Atropisomeric Heterobiaryls. Synthesis. 2022;54:2119–2132. doi: 10.1055/s-0040-1719896. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

