Methylglyoxal in the Brain: From Glycolytic Metabolite to Signalling Molecule
- PMID: 36432007
- PMCID: PMC9696358
- DOI: 10.3390/molecules27227905
Methylglyoxal in the Brain: From Glycolytic Metabolite to Signalling Molecule
Abstract
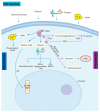
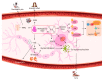
Advances in molecular biology technology have piqued tremendous interest in glycometabolism and bioenergetics in homeostasis and neural development linked to ageing and age-related diseases. Methylglyoxal (MGO) is a by-product of glycolysis, and it can covalently modify proteins, nucleic acids, and lipids, leading to cell growth inhibition and, eventually, cell death. MGO can alter intracellular calcium homeostasis, which is a major cell-permeant precursor to advanced glycation end-products (AGEs). As side-products or signalling molecules, MGO is involved in several pathologies, including neurodevelopmental disorders, ageing, and neurodegenerative diseases. In this review, we demonstrate that MGO (the metabolic side-product of glycolysis), the GLO system, and their analogous relationship with behavioural phenotypes, epigenetics, ageing, pain, and CNS degeneration. Furthermore, we summarise several therapeutic approaches that target MGO and the glyoxalase (GLO) system in neurodegenerative diseases.
Keywords: behavioural phenotypes; bioenergetics; glyoxalase; homeostasis; methylglyoxal; neurodegenerative; side-product.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Shin M.J., Kim D.W., Lee Y.P., Ahn E.H., Jo H.S., Kim D.S., Kwon O.S., Kang T.C., Cho Y.J., Park J., et al. Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic. Biol. Med. 2014;67:195–210. doi: 10.1016/j.freeradbiomed.2013.10.815. - DOI - PubMed
-
- Bilova T., Paudel G., Shilyaev N., Schmidt R., Brauch D., Tarakhovskaya E., Milrud S., Smolikova G., Tissier A., Vogt T., et al. Global proteomic analysis of advanced glycation end products in the Arabidopsis proteome provides evidence for age-related glycation Hotspots. J. Biol. Chem. 2017;22:15758–15776. doi: 10.1074/jbc.M117.794537. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

