Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5- a]Pyridine-3-phosphonates
- PMID: 36432015
- PMCID: PMC9694626
- DOI: 10.3390/molecules27227913
Oxidative [3+2]Cycloaddition of Alkynylphosphonates with Heterocyclic N-Imines: Synthesis of Pyrazolo[1,5- a]Pyridine-3-phosphonates
Abstract
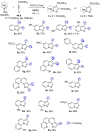
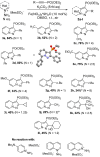
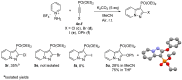
A series of pyrazolo[1,5-a]pyridine-3-ylphosphonates were prepared with moderate to good yields by the oxidative [3+2]cycloaddition of 2-subtituted ethynylphosphonates with in situ generated pyridinium-N-imines and their annulated analogs. 2-Aliphatic and 2-Ph acetylenes demonstrate low activity, and the corresponding pyrazolopyridines were achieved with a moderate yield in the presence of 10 mol% Fe(NO3)3·9H2O. At the same time, tetraethyl ethynylbisphosphonate, diethyl 2-TMS- and 2-OPh-ethynylphosphonates possess much greater reactivity and the corresponding pyrazolo[1,5-a]pyridines, and their annulated derivatives were obtained with good to excellent yields without any catalyst. 2-Halogenated ethynylphosphonates also readily reacted with pyridinium-N-imines, forming complex mixtures containing poor amounts of 2-halogenated pyrazolopyridines.
Keywords: alkynes; cycloaddition; heterocycles; iron; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nakao S., Nogami M., Iwatani M., Imaeda T., Ito M., Tanaka T., Tawada M., Endo S., Cary D.R., Ohori M., et al. Identification of a Selective DDX3X Inhibitor with Newly Developed Quantitative High-Throughput RNA Helicase Assays. Biochem. Biophys. Res. Commun. 2020;523:795–801. doi: 10.1016/j.bbrc.2019.12.094. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

