Novel Copper Oxide Bio-Nanocrystals to Target Outer Membrane Lectin of Vancomycin-Resistant Enterococcus faecium (VREfm): In Silico, Bioavailability, Antimicrobial, and Anticancer Potential
- PMID: 36432057
- PMCID: PMC9696412
- DOI: 10.3390/molecules27227957
Novel Copper Oxide Bio-Nanocrystals to Target Outer Membrane Lectin of Vancomycin-Resistant Enterococcus faecium (VREfm): In Silico, Bioavailability, Antimicrobial, and Anticancer Potential
Abstract
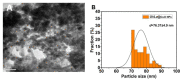
In present study, we used Olea europaea leaf extract to biosynthesize in situ Copper Oxide nanocrystals (CuO @OVLe NCs) with powerful antibacterial and anti-cancer capabilities. Physio-chemical analyses, such as UV/Vis, FTIR, XRD, EDX, SEM, and TEM, were applied to characterize CuO @OVLe NCs. The UV/Vis spectrum demonstrated a strong peak at 345 nm. Furthermore, FTIR, XRD, and EDX validated the coating operation's contact with colloidal CuO @OVLe NCs. According to TEM and SEM analyses, CuO @OVLe NCs exhibited a spherical shape and uniform distribution of size with aggregation, for an average size of ~75 nm. The nanoparticles demonstrated a considerable antibacterial effect against E. faecium bacterial growth, as well as an increased inhibition rate in a dose-dependent manner on the MCF-7, PC3, and HpeG2 cancer cell lines and a decreased inhibition rate on WRL-68. Molecular docking and MD simulation were used to demonstrate the high binding affinity of a ligand (Oleuropein) toward the lectin receptor complex of the outer membrane to vancomycin-resistant E. faecium (VREfm) via amino acids (Leu 195, Thr 288, His 165, and Ser 196). Hence, our results expand the accessibility of OVLe's bioactive components as a promising natural source for the manufacture of physiologically active components and the creation of green biosynthesis of metal nanocrystals.
Keywords: Enterococcus faecium; Olea europaea; anti-cancer; antimicrobial; green biosynthesis; molecular docking; molecular dynamic simulation; nanocrystal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Clinically Applicable System for Rapidly Predicting Enterococcus faecium Susceptibility to Vancomycin.Microbiol Spectr. 2021 Dec 22;9(3):e0091321. doi: 10.1128/Spectrum.00913-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756065 Free PMC article.
-
Fabrication of chitosan and Trianthema portulacastrum mediated copper oxide nanoparticles: Antimicrobial potential against MDR bacteria and biological efficacy for antioxidant, antidiabetic and photocatalytic activities.Int J Biol Macromol. 2023 Jul 1;242(Pt 3):124954. doi: 10.1016/j.ijbiomac.2023.124954. Epub 2023 May 19. Int J Biol Macromol. 2023. PMID: 37211075
-
Vancomycin-resistant Enterococcus faecium in Algeria: phenotypic and genotypic characterization of clinical isolates.J Infect Dev Ctries. 2021 Jan 31;15(1):95-101. doi: 10.3855/jidc.12482. J Infect Dev Ctries. 2021. PMID: 33571151
-
Genomics of vancomycin-resistant Enterococcus faecium.Microb Genom. 2019 Jul;5(7):e000283. doi: 10.1099/mgen.0.000283. Epub 2019 Jul 22. Microb Genom. 2019. PMID: 31329096 Free PMC article. Review.
-
Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future.J Glob Antimicrob Resist. 2019 Mar;16:36-47. doi: 10.1016/j.jgar.2018.08.016. Epub 2018 Aug 24. J Glob Antimicrob Resist. 2019. PMID: 30149193 Review.
Cited by
-
Metallic nanoparticles: a promising novel therapeutic tool against antimicrobial resistance and spread of superbugs.Biometals. 2025 Feb;38(1):55-88. doi: 10.1007/s10534-024-00647-5. Epub 2024 Oct 24. Biometals. 2025. PMID: 39446237 Review.
-
Biogenic silver nanoparticles eradicate of Pseudomonas aeruginosa and Methicillin-resistant Staphylococcus aureus (MRSA) isolated from the sputum of COVID-19 patients.Front Microbiol. 2023 Apr 6;14:1142646. doi: 10.3389/fmicb.2023.1142646. eCollection 2023. Front Microbiol. 2023. PMID: 37143540 Free PMC article.
-
Green synthesis of silver nanoparticles from yam leaves: characterisation and in vitro efficacy against three phytopathogenic bacteria.Antonie Van Leeuwenhoek. 2025 Aug 9;118(9):126. doi: 10.1007/s10482-025-02136-2. Antonie Van Leeuwenhoek. 2025. PMID: 40782166
References
-
- Khalifa S.A.M., Shedid E.S., Saied E.M., Jassbi A.R., Jamebozorgi F.H., Rateb M.E., Du M., Abdel-Daim M.M., Kai G.-Y., Al-Hammady M.A.M., et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs. 2021;19:241. doi: 10.3390/md19050241. - DOI - PMC - PubMed
-
- Koch-Edelmann S., Banhart S., Saied E.M., Rose L., Aeberhard L., Laue M., Doellinger J., Arenz C., Heuer D. The cellular ceramide transport protein CERT promotes Chlamydia psittaci infection and controls bacterial sphingolipid uptake. Cell. Microbiol. 2017;19:e12752. doi: 10.1111/cmi.12752. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

