A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates
- PMID: 36432089
- PMCID: PMC9696695
- DOI: 10.3390/molecules27227990
A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates
Abstract
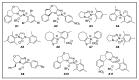
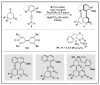
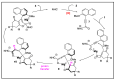
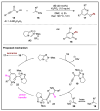
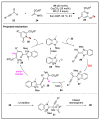
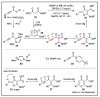
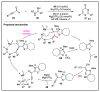
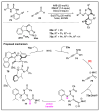
N-heterocyclic carbene (NHC) has been widely used as an organocatalyst for both umpolung and non-umpolung chemistry. Previous works mainly focus on species including Breslow intermediate, azolium enolate intermediate, homoenolate intermediate, alkenyl acyl azolium intermediate, etc. Notably, the NHC-bound alkynyl acyl azolium has emerged as an effective intermediate to access functionalized cyclic molecular skeleton until very recently. In this review, we summarized the generation and reactivity of the NHC-bound alkynyl acyl azolium intermediates, which covers the efforts and advances in the synthesis of achiral and axially chiral cyclic scaffolds via the NHC-bound alkynyl acyl azolium intermediates. In particular, the mechanism related to this intermediate is discussed in detail.
Keywords: N-heterocyclic carbene; alkynyl acyl azolium; annulation; intermediate; mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
N-Heterocyclic Carbene (NHC)-Catalyzed Transformations Involving Azolium Enolates.Chem Rec. 2022 Aug;22(8):e202200054. doi: 10.1002/tcr.202200054. Epub 2022 May 13. Chem Rec. 2022. PMID: 35562645 Review.
-
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations.Angew Chem Int Ed Engl. 2018 Apr 3;57(15):3862-3873. doi: 10.1002/anie.201709684. Epub 2018 Mar 9. Angew Chem Int Ed Engl. 2018. PMID: 29136320 Review.
-
Acyl Donor Intermediates in N-Heterocyclic Carbene Catalysis: Acyl Azolium or Azolium Enolate?Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4507-4511. doi: 10.1002/anie.202010348. Epub 2021 Jan 18. Angew Chem Int Ed Engl. 2021. PMID: 33140529 Free PMC article.
-
Reductive N-Heterocyclic Carbene Catalysis via Hydride Transfer: Generating Homoenolates from Unsaturated Esters.Org Lett. 2025 Jun 6;27(22):5799-5805. doi: 10.1021/acs.orglett.5c01604. Epub 2025 May 27. Org Lett. 2025. PMID: 40420662
-
NHC-Activations on α-, β-, γ-, and Beyond.Chem Rec. 2023 Jul;23(7):e202200279. doi: 10.1002/tcr.202200279. Epub 2023 Mar 14. Chem Rec. 2023. PMID: 36916715 Review.
Cited by
-
Organocatalytic atroposelective de novo construction of monoaxially and 1,4-diaxially chiral fused uracils with potential antitumor activity.Chem Sci. 2025 Mar 28;16(18):7876-7883. doi: 10.1039/d5sc00452g. eCollection 2025 May 7. Chem Sci. 2025. PMID: 40191121 Free PMC article.
References
-
- Ukai T., Tanaka R., Dokawa T. A new catalyst for acyloin condensation. J. Pharm. Soc. Jpn. 1943;63:296–300.
-
- Breslow R. On the mechanism of thiamine action. Iv.1 evidence from studies on model systems. J. Am. Chem. Soc. 1958;80:3719–3726. doi: 10.1021/ja01547a064. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

