A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates
- PMID: 36432089
- PMCID: PMC9696695
- DOI: 10.3390/molecules27227990
A Review on Generation and Reactivity of the N-Heterocyclic Carbene-Bound Alkynyl Acyl Azolium Intermediates
Abstract
N-heterocyclic carbene (NHC) has been widely used as an organocatalyst for both umpolung and non-umpolung chemistry. Previous works mainly focus on species including Breslow intermediate, azolium enolate intermediate, homoenolate intermediate, alkenyl acyl azolium intermediate, etc. Notably, the NHC-bound alkynyl acyl azolium has emerged as an effective intermediate to access functionalized cyclic molecular skeleton until very recently. In this review, we summarized the generation and reactivity of the NHC-bound alkynyl acyl azolium intermediates, which covers the efforts and advances in the synthesis of achiral and axially chiral cyclic scaffolds via the NHC-bound alkynyl acyl azolium intermediates. In particular, the mechanism related to this intermediate is discussed in detail.
Keywords: N-heterocyclic carbene; alkynyl acyl azolium; annulation; intermediate; mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


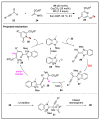
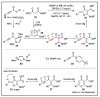
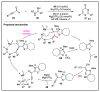



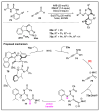
References
-
- Ukai T., Tanaka R., Dokawa T. A new catalyst for acyloin condensation. J. Pharm. Soc. Jpn. 1943;63:296–300.
-
- Breslow R. On the mechanism of thiamine action. Iv.1 evidence from studies on model systems. J. Am. Chem. Soc. 1958;80:3719–3726. doi: 10.1021/ja01547a064. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

