Tricyano-Methylene-Pyridine Based High-Performance Aggregation-Induced Emission Photosensitizer for Imaging and Photodynamic Therapy
- PMID: 36432090
- PMCID: PMC9697965
- DOI: 10.3390/molecules27227981
Tricyano-Methylene-Pyridine Based High-Performance Aggregation-Induced Emission Photosensitizer for Imaging and Photodynamic Therapy
Abstract
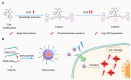
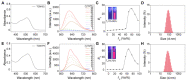
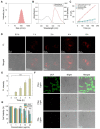
Photosensitizers equipped with high reactive oxygen species (ROS) generation capability and bright emission are essential for accurate tumor imaging and precise photodynamic therapy (PDT). However, traditional aggregation-caused quenching (ACQ) photosensitizers cannot simultaneously produce desirable ROS and bright fluorescence, resulting in poor image-guided therapy effect. Herein, we report an aggregation-induced emission (AIE) photosensitizer TCM-Ph with a strong donor-π-acceptor (D-π-A) structure, which greatly separates the HOMO-LUMO distribution and reduces the ΔEST, thereby increasing the number of triplet excitons and producing more ROS. The AIE photosensitizer TCM-Ph has bright near-infrared emission, as well as a higher ROS generation capacity than the commercial photosensitizers Bengal Rose (RB) and Chlorine e6 (Ce6), and can effectively eliminate cancer cells under image guidance. Therefore, the AIE photosensitizer TCM-Ph has great potential to replace the commercial photosensitizers.
Keywords: ROS generation; aggregation-induced emission; bioimaging; photosensitizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
AIE-active luminogens as highly efficient free-radical ROS photogenerator for image-guided photodynamic therapy.Chem Sci. 2022 Feb 23;13(12):3599-3608. doi: 10.1039/d2sc00067a. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432854 Free PMC article.
-
Highly Efficient Near-Infrared Photosensitizers with Aggregation-Induced Emission Characteristics: Rational Molecular Design and Photodynamic Cancer Cell Ablation.ACS Appl Bio Mater. 2021 Jun 21;4(6):5231-5239. doi: 10.1021/acsabm.1c00398. Epub 2021 May 19. ACS Appl Bio Mater. 2021. PMID: 35007005
-
AIE-Active Photosensitizers: Manipulation of Reactive Oxygen Species Generation and Applications in Photodynamic Therapy.Biosensors (Basel). 2022 May 18;12(5):348. doi: 10.3390/bios12050348. Biosensors (Basel). 2022. PMID: 35624649 Free PMC article. Review.
-
Highly Efficient Multifunctional Organic Photosensitizer with Aggregation-Induced Emission for In Vivo Bioimaging and Photodynamic Therapy.ACS Appl Mater Interfaces. 2021 Nov 24;13(46):54783-54793. doi: 10.1021/acsami.1c17476. Epub 2021 Nov 11. ACS Appl Mater Interfaces. 2021. PMID: 34763423
-
Design and structural regulation of AIE photosensitizers for imaging-guided photodynamic anti-tumor application.Biomater Sci. 2022 Aug 9;10(16):4443-4457. doi: 10.1039/d2bm00864e. Biomater Sci. 2022. PMID: 35789348 Review.
Cited by
-
An azo substituted quinoline-malononitrile enzyme-activable aggregation-induced emission nanoprobe for hypoxia imaging.Smart Mol. 2024 Sep 4;3(2):e20240028. doi: 10.1002/smo.20240028. eCollection 2025 Jun. Smart Mol. 2024. PMID: 40671929 Free PMC article.
-
Study on the Construction and Anti-Tumor Effect of aPDL1/aMUC1 Double Antibody Modification of Doxorubicin Liposome.ACS Omega. 2025 Mar 3;10(10):10107-10121. doi: 10.1021/acsomega.4c08564. eCollection 2025 Mar 18. ACS Omega. 2025. PMID: 40124041 Free PMC article.
References
-
- Zhang Y., Zhao X., Li Y., Wang X., Wang Q., Lu H., Zhu L. A fluorescent photosensitizer with far red/near-infrared aggregation-induced emission for imaging and photodynamic killing of bacteria. Dyes Pigments. 2019;165:53–57. doi: 10.1016/j.dyepig.2019.02.019. - DOI
-
- Xu W., Zhang Z., Kang M., Guo H., Li Y., Wen H., Lee M.M.S., Wang Z., Kwok R.T.K., Lam J.W.Y., et al. Making the best use of excited-state energy: Multimodality theranostic systems based on second near-infrared (NIR-II) aggregation-induced emission luminogens (AIEgens) ACS Mater. Lett. 2020;2:1033–1040. doi: 10.1021/acsmaterialslett.0c00263. - DOI
-
- Wang D., Su H., Kwok R.T.K., Shan G., Leung A.C.S., Lee M.M.S., Sung H.H.Y., Williams I.D., Lam J.W.Y., Tang B.Z. Facile synthesis of red/NIR AIE luminogens with simple structures, bright emissions, and high photostabilities, and their applications for specific imaging of lipid droplets and image-guided photodynamic therapy. Adv. Funct. Mater. 2017;27:1704039. doi: 10.1002/adfm.201704039. - DOI
MeSH terms
Substances
Grants and funding
- 22222803/National Natural Science Foundation of China
- 91959202/National Natural Science Foundation of China
- 21974047/National Natural Science Foundation of China
- 2018SHZDZX03/Shanghai Municipal Science and Technology Major Project
- 2021YFA0910000/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

