4-(Aryl)-Benzo[4,5]imidazo[1,2- a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations
- PMID: 36432130
- PMCID: PMC9698514
- DOI: 10.3390/molecules27228029
4-(Aryl)-Benzo[4,5]imidazo[1,2- a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations
Abstract
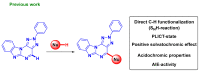
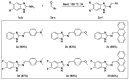
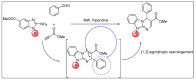
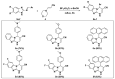
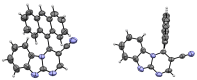
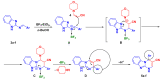
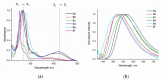
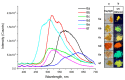
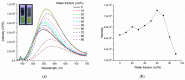
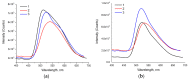
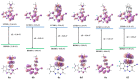
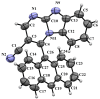
A series of novel 4-(aryl)-benzo[4,5]imidazo[1,2-a]pyrimidine-3-carbonitriles were obtained through the Povarov (aza-Diels-Alder) and oxidation reactions, starting from benzimidazole-2-arylimines. Based on the literature data and X-ray diffraction analysis, it was discovered that during the Povarov reaction, [1,3] sigmatropic rearrangement leading to dihydrobenzimidazo[1,2-a]pyrimidines took place. The structures of all the obtained compounds were confirmed based on the data from 1H- and 13C-NMR spectroscopy, IR spectroscopy, and elemental analysis. For all the obtained compounds, their photophysical properties were studied. In all the cases, a positive emission solvatochromism with Stokes shifts from 120 to 180 nm was recorded. Aggregation-Induced Emission (AIE) has been illustrated for compound 6c using different water fractions (fw) in THF. The compounds 6c and 6f demonstrated changes in emission maxima or/and intensities after mechanical stimulation.
Keywords: Povarov reaction; aggregation-induced emission; aza-Diels–Alder reaction; benzimidazole; fluorescence; mechanochromic properties; oxidation; pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Synthesis, Spectroscopic Characterization and DFT/TD-DFT Calculations of new Fluorescent Derivatives of Imidazo[4',5':3,4]Benzo[c]Isoxazole.J Fluoresc. 2016 Mar;26(2):513-9. doi: 10.1007/s10895-015-1736-5. Epub 2015 Dec 9. J Fluoresc. 2016. PMID: 26650817
-
ICT and AIE Characteristics Two Cyano-Functionalized Probes and Their Photophysical Properties, DFT Calculations, Cytotoxicity, and Cell Imaging Applications.Molecules. 2020 Jan 29;25(3):585. doi: 10.3390/molecules25030585. Molecules. 2020. PMID: 32013190 Free PMC article.
-
Synthesis, Spectral Studies and Quantum-Chemical Investigations on the Powerful Fluorophores: Imidazo[4,5-a]acridines.J Fluoresc. 2015 Sep;25(5):1235-43. doi: 10.1007/s10895-015-1611-4. Epub 2015 Jul 16. J Fluoresc. 2015. PMID: 26179073
-
Excited-State Intramolecular Proton Transfer Dyes with Dual-State Emission Properties: Concept, Examples and Applications.Molecules. 2022 Apr 10;27(8):2443. doi: 10.3390/molecules27082443. Molecules. 2022. PMID: 35458640 Free PMC article. Review.
-
Zinc (II) and AIEgens: The "Clip Approach" for a Novel Fluorophore Family. A Review.Molecules. 2021 Jul 9;26(14):4176. doi: 10.3390/molecules26144176. Molecules. 2021. PMID: 34299451 Free PMC article. Review.
Cited by
-
Microwave-assisted synthesis and functionalization of 2-arylimidazo[1,2-a]pyrimidin-5(8H)-ones.RSC Adv. 2024 Jul 15;14(31):22368-22373. doi: 10.1039/d4ra03948c. eCollection 2024 Jul 12. RSC Adv. 2024. PMID: 39010922 Free PMC article.
-
Isomerization of pirazolopyrimidines to pyrazolopyridines by ring-opening/closing reaction in aqueous NaOH.RSC Adv. 2025 Jan 22;15(3):2078-2085. doi: 10.1039/d4ra06345g. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39845108 Free PMC article.
-
Synthesis of Heteroaromatic Compounds.Molecules. 2023 Apr 19;28(8):3563. doi: 10.3390/molecules28083563. Molecules. 2023. PMID: 37110797 Free PMC article.
References
-
- Alqarni S., Cooper L., Galvan Achi J., Bott R., Sali V.K., Brown A., Santarsiero B.D., Krunic A., Manicassamy B., Peet N.P., et al. Synthesis, Optimization, and Structure–Activity Relationships of Imidazo[1,2-a]Pyrimidines as Inhibitors of Group 2 Influenza A Viruses. J. Med. Chem. 2022;65:14104–14120. doi: 10.1021/acs.jmedchem.2c01329. - DOI - PubMed
-
- Massari S., Bertagnin C., Pismataro M.C., Donnadio A., Nannetti G., Felicetti T., Di Bona S., Nizi M.G., Tensi L., Manfroni G., et al. Synthesis and Characterization of 1,2,4-Triazolo[1,5-a]Pyrimidine-2-Carboxamide-Based Compounds Targeting the PA-PB1 Interface of Influenza A Virus Polymerase. Eur. J. Med. Chem. 2021;209:112944. doi: 10.1016/j.ejmech.2020.112944. - DOI - PMC - PubMed
-
- Romagnoli R., Oliva P., Prencipe F., Manfredini S., Budassi F., Brancale A., Ferla S., Hamel E., Corallo D., Aveic S., et al. Design, Synthesis and Biological Investigation of 2-Anilino Triazolopyrimidines as Tubulin Polymerization Inhibitors with Anticancer Activities. Pharmaceuticals. 2022;15:1031. doi: 10.3390/ph15081031. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

