Thermodynamics and Kinetics of Electron Transfer of Electrode-Immobilized Small Laccase from Streptomyces coelicolor
- PMID: 36432180
- PMCID: PMC9692349
- DOI: 10.3390/molecules27228079
Thermodynamics and Kinetics of Electron Transfer of Electrode-Immobilized Small Laccase from Streptomyces coelicolor
Abstract
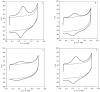
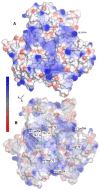
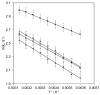
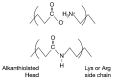
The thermodynamic and kinetic properties for heterogeneous electron transfer (ET) were measured for the electrode-immobilized small laccase (SLAC) from Streptomyces coelicolor subjected to different electrostatic and covalent protein-electrode linkages, using cyclic voltammetry. Once immobilized electrostatically onto a gold electrode using mixed carboxyl- and hydroxy-terminated alkane-thiolate SAMs or covalently exploiting the same SAM subjected to N-hydroxysuccinimide+1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (NHS-EDC) chemistry, the SLAC-electrode electron flow occurs through the T1 center. The E°' values (from +0.2 to +0.1 V vs. SHE at pH 7.0) are lower by more than 0.2 V compared to the protein either in solution or immobilized with different anchoring strategies using uncharged SAMs. For the present electrostatic and covalent binding, this effect can, respectively, be ascribed to the negative charge of the SAM surfaces and to deletion of the positive charge of Lys/Arg residues due to amide bond formation which both selectively stabilize the more positively charged oxidized SLAC. Observation of enthalpy/entropy compensation within the series indicates that the immobilized proteins experience different reduction-induced solvent reorganization effects. The E°' values for the covalently attached SLAC are sensitive to three acid base equilibria, with apparent pKa values of pKa1ox = 5.1, pKa1red = 7.5, pKa2ox = 8.4, pKa2red = 10.9, pKa2ox = 8.9, pKa2red = 11.3 possibly involving one residue close to the T1 center and two residues (Lys and/or Arg) along with moderate protein unfolding, respectively. Therefore, the E°' value of immobilized SLAC turns out to be particularly sensitive to the anchoring mode and medium conditions.
Keywords: electron transfer; protein voltammetry; protein-electrode linkage; redox thermodynamics; self-assembled monolayers; small laccase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Oxygen-reducing enzyme cathodes produced from SLAC, a small laccase from Streptomyces coelicolor.Biosens Bioelectron. 2008 Mar 14;23(8):1229-35. doi: 10.1016/j.bios.2007.11.004. Epub 2007 Nov 13. Biosens Bioelectron. 2008. PMID: 18096378
-
Effects of mutational (Lys to Ala) surface charge changes on the redox properties of electrode-immobilized cytochrome c.J Phys Chem B. 2007 Aug 30;111(34):10281-7. doi: 10.1021/jp0730343. Epub 2007 Aug 9. J Phys Chem B. 2007. PMID: 17685644
-
Channeling of electrons within SLAC, the small laccase from Streptomyces coelicolor.Faraday Discuss. 2011;148:161-71; discussion 207-28. doi: 10.1039/c002585b. Faraday Discuss. 2011. PMID: 21322483
-
Direct bio-electrocatalysis of O2 reduction by Streptomyces coelicolor laccase orientated at promoter-modified graphite electrodes.Chemphyschem. 2013 Jul 22;14(10):2112-24. doi: 10.1002/cphc.201300069. Epub 2013 Apr 15. Chemphyschem. 2013. PMID: 23589501
-
Recent developments and applications of immobilized laccase.Biotechnol Adv. 2013 Dec;31(8):1808-25. doi: 10.1016/j.biotechadv.2012.02.013. Epub 2012 Feb 28. Biotechnol Adv. 2013. PMID: 22398306 Review.
References
-
- Yoshida H. LXIII.—Chemistry of Lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 1883;43:472–486. doi: 10.1039/CT8834300472. - DOI
-
- Lontie R. In: Copper Proteins and Copper Enzymes. Lontie R., editor. CRC Press; Boca Raton, FL, USA: 2018.
-
- Yaropolov A.I., Skorobogat’ko O.V., Vartanov S.S., Varfolomeyev S.D. Laccase. Appl. Biochem. Biotechnol. 1994;49:257–280. doi: 10.1007/BF02783061. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

