Two Theorems and Important Insight on How the Preferred Mechanism of Free Radical Scavenging Cannot Be Settled. Comment on Pandithavidana, D.R.; Jayawardana, S.B. Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights. Molecules 2019, 24, 1646
- PMID: 36432191
- PMCID: PMC9692462
- DOI: 10.3390/molecules27228092
Two Theorems and Important Insight on How the Preferred Mechanism of Free Radical Scavenging Cannot Be Settled. Comment on Pandithavidana, D.R.; Jayawardana, S.B. Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights. Molecules 2019, 24, 1646
Abstract
Totally ignoring that the five enthalpies of reaction—bond dissociation enthalpy (BDE), adiabatic ionization potential (IP), proton dissociation enthalpy (PDE), proton affinity (PA), and electron transfer enthalpy (ETE)—characterizing the three free radical scavenging mechanisms—direct hydrogen atom transfer (HAT), sequential electron transfer proton transfer (SET-PT), and stepwise proton loss electron transfer (SPLET)—are not independent of each other, a recent publication on the antioxidant activity of dietary vitamins compared various vitamins and “found” different quantities, which should be strictly equal by virtue of energy conservation. Aiming to clarify this point, as well as to avoid such mistakes in future studies and to unravel errors in the previous literature, in the present paper we formulate two theorems that any sound results on antioxidation should obey. The first theorem states that the sums of the enthalpies characterizing the individual steps of SET-PT and SPLET are equal: IP+PDE = PA+ETE (=H2). This is a mathematical identity emerging from the fact that both the reactants and the final products of SET-PT and SPLET are chemically identical. The second theorem, which is also a mathematical identity, states that H2 − BDE = IPH > 0, where IPH is the ionization potential of the H-atom in the medium (e.g., gas or solvent) considered. Due to their general character, these theorems may/should serve as necessary sanity tests for any results on antioxidant activity, whatever the method employed in their derivation. From a more general perspective, they should represent a serious word of caution regarding attempts to assign the preferred free radical scavenging pathway based merely on thermochemical descriptors.
Keywords: BDE; ETE; HAT; IP; PA; PDE; SET-PT; SPLET; antioxidant mechanisms; quantum chemistry; radical scavenging activity; thermochemistry.
Conflict of interest statement
The author declares no conflict of interest.
Figures
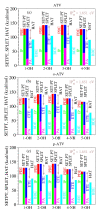

Comment on
- Molecules. 24:1646.
References
-
- Sies H. Oxidative Stress. Academic Press; Cambridge, MA, USA: 1985. pp. 1–501.
-
- Sies H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. 1986;25:1058–1071. doi: 10.1002/anie.198610581. - DOI
-
- Sies H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018;7:122–126. doi: 10.1016/j.cotox.2018.01.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

