Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene
- PMID: 36432303
- PMCID: PMC9697087
- DOI: 10.3390/nano12224017
Green Removal of DUV-Polarity-Modified PMMA for Wet Transfer of CVD Graphene
Abstract
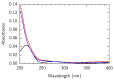
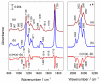
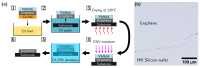
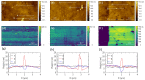
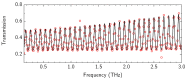
To fabricate graphene-based high-frequency electronic and optoelectronic devices, there is a high demand for scalable low-contaminated graphene with high mobility. Graphene synthesized via chemical vapor deposition (CVD) on copper foil appears promising for this purpose, but residues from the polymethyl methacrylate (PMMA) layer, used for the wet transfer of CVD graphene, drastically affect the electrical properties of graphene. Here, we demonstrate a scalable and green PMMA removal technique that yields high-mobility graphene on the most common technologically relevant silicon (Si) substrate. As the first step, the polarity of the PMMA was modified under deep-UV irradiation at λ = 254 nm, due to the formation of ketones and aldehydes of higher polarity, which simplifies hydrogen bonding in the step of its dissolution. Modification of PMMA polarity was confirmed by UV and FTIR spectrometry and contact angle measurements. Consecutive dissolution of DUV-exposed PMMA in an environmentally friendly, binary, high-polarity mixture of isopropyl alcohol/water (more commonly alcohol/water) resulted in the rapid and complete removal of DUV-exposed polymers without the degradation of graphene properties, as low-energy exposure does not form free radicals, and thus the released graphene remained intact. The high quality of graphene after PMMA removal was confirmed by SEM, AFM, Raman spectrometry, and by contact and non-contact electrical conductivity measurements. The removal of PMMA from graphene was also performed via other common methods for comparison. The charge carrier mobility in graphene films was found to be up to 6900 cm2/(V·s), demonstrating a high potential of the proposed PMMA removal method in the scalable fabrication of high-performance electronic devices based on CVD graphene.
Keywords: DUV; PMMA; THz-TDS; graphene.
Conflict of interest statement
N.A. and I.K. are inventors on a provision patent application filed by the Valstybinis mokslinių tyrimų institutas Fizinių ir technologijos mokslų centras (no. EP3936476A1, published 12 January 2022). The other authors declare no conflict of interest.
Figures



References
-
- Geim A.K., Novoselov K.S., Falko V.I., Colombo L., Gellert P.R., Schwab M.G., Kim K., Ferrari A.C., Bonaccorso F., Falko V.I., et al. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nature. 2012;7:192–200. doi: 10.1039/C4NR01600A. - DOI
-
- Bolotin K.I., Sikes K.J., Jiang Z., Klima M., Fudenberg G., Hone J., Kim P., Stormer H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008;146:351–355. doi: 10.1016/j.ssc.2008.02.024. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

