Ag-Activated Metal-Organic Framework with Peroxidase-like Activity Synergistic Ag+ Release for Safe Bacterial Eradication and Wound Healing
- PMID: 36432344
- PMCID: PMC9696893
- DOI: 10.3390/nano12224058
Ag-Activated Metal-Organic Framework with Peroxidase-like Activity Synergistic Ag+ Release for Safe Bacterial Eradication and Wound Healing
Abstract
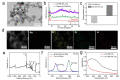
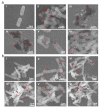
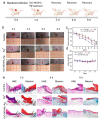
Silver nanoparticles (Ag NPs), a commonly used antibacterial nanomaterial, exhibit broad-spectrum antibacterial activity to combat drug-resistant bacteria. However, the Ag NPs often causes a low availability and high toxicity to living bodies due to their easy aggregation and uncontrolled release of Ag+ in the bacterial microenvironment. Here, we report a porous metal-organic framework (MOF)-based Zr-2-amin-1,4-NH2-benzenedicarboxylate@Ag (denoted as UiO-66-NH2-Ag) nanocomposite using an in-situ immobilization strategy where Ag NPs were fixed on the UiO-66-NH2 for improving the dispersion and utilization of Ag NPs. As a result, the reduced use dose of Ag NPs largely improves the biosafety of the UiO-66-NH2-Ag. Meanwhile, after activation by the Ag NPs, the UiO-66-NH2-Ag can act as nanozyme with high peroxidase (POD)-like activity to efficiently catalyze the decomposition of H2O2 to extremely toxic hydroxyl radicals (·OH) in the bacterial microenvironment. Simultaneously, the high POD-like activity synergies with the controllable Ag+ release leads to enhanced reactive oxygen species (ROS) generation, facilitating the death of resistant bacteria. This synergistic antibacterial strategy enables the low concentration (12 μg/mL) of UiO-66-NH2-Ag to achieve highly efficient inactivation of ampicillin-resistant Escherichia coli (AmprE. coli) and endospore-forming Bacillus subtilis (B. subtilis). In vivo results illustrate that the UiO-66-NH2-Ag nanozyme has a safe and accelerated bacteria-infected wound healing.
Keywords: antibacterial; metal−organic framework; nanozyme; silver ions release; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang F., Fang R.H., Luk B.T., Hu C.J., Thamphiwatana S., Dehaini D., Angsantikul P., Kroll A.V., Pang Z., Gao W., et al. Nanoparticle-Based Antivirulence Vaccine for The Management of Methicillin-Resistant Staphylococcus Aureus Skin Infection. Adv. Funct. Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

