On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive
- PMID: 36432359
- PMCID: PMC9693044
- DOI: 10.3390/nano12224075
On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive
Abstract
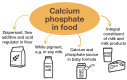
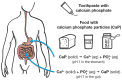
The human body needs calcium and phosphate as essential nutrients to grow bones and teeth, but they are also necessary for many other biochemical purposes (e.g., the biosynthesis of phospholipids, adenosine triphosphate, ATP, or DNA). The use of solid calcium phosphate in particle form as a food additive is reviewed and discussed in terms of bioavailability and its safety after ingestion. The fact that all calcium phosphates, such as hydroxyapatite and tricalcium phosphate, are soluble in the acidic environment of the stomach, regardless of the particle size or phase, means that they are present as dissolved ions after passing through the stomach. These dissolved ions cannot be distinguished from a mixture of calcium and phosphate ions that were ingested separately, e.g., from cheese or milk together with soft drinks or meat. Milk, including human breast milk, is a natural source of calcium and phosphate in which calcium phosphate is present as nanoscopic clusters (nanoparticles) inside casein (protein) micelles. It is concluded that calcium phosphates are generally safe as food additives, also in baby formula.
Keywords: calcium phosphate; food additives; microparticles; nanoparticles.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures
References
-
- Cai Y., Tang R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008;18:3775–3787. doi: 10.1039/b805407j. - DOI
-
- Elliott J.C. Calcium phosphate biominerals. Rev. Mineral. 2002;48:427–453. doi: 10.2138/rmg.2002.48.11. - DOI
-
- Dorozhkin S.V. Calcium orthophosphates in nature, biology and medicine. Materials. 2009;2:399–498. doi: 10.3390/ma2020399. - DOI
-
- Fabritius H.O., Enax J., Meyer F. Eine Reise ins Innere unserer Zähne / A journey into our teeth. Titus Verlag; Bielefeld, Germany: 2021.
Publication types
LinkOut - more resources
Full Text Sources

