Puerarin Reduces Oxidative Damage and Photoaging Caused by UVA Radiation in Human Fibroblasts by Regulating Nrf2 and MAPK Signaling Pathways
- PMID: 36432411
- PMCID: PMC9694396
- DOI: 10.3390/nu14224724
Puerarin Reduces Oxidative Damage and Photoaging Caused by UVA Radiation in Human Fibroblasts by Regulating Nrf2 and MAPK Signaling Pathways
Abstract
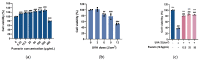
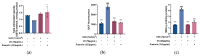
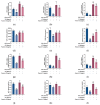
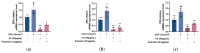
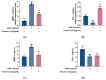
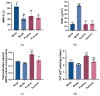
Fibroblasts account for more than 95% of dermal cells maintaining dermal structure and function. However, UVA penetrates the dermis and causes oxidative stress that damages the dermis and accelerates skin aging. Puerarin, the main active ingredient of Puerariae lobata, has been demonstrated to withstand oxidative stress caused by a variety of factors. However, there are limited findings on whether puerarin protects fibroblasts from UVA-induced oxidative stress damage. The effects of puerarin on human skin fibroblasts (HSF) under UVA-induced oxidative stress were investigated in this study. It is found that puerarin upregulates antioxidant enzymes' mRNA expression level and their content through modulating the KEAP1-Nrf2/ARE signaling pathway, thus improving cell antioxidant capacity and successfully eliminating UVA-induced reactive oxygen species (ROS) and lipid oxidation product malondialdehyde (MDA). Additionally, puerarin blocks the overexpression of human extracellular signal-regulated kinase (ERK), human c-Jun amino-terminal kinase (JNK), and P38, which downregulates matrix metalloproteinase 1 (MMP-1) expression and increases type I collagen (COL-1) expression. Moreover, preliminary research on mouse skin suggests that puerarin can hydrate, moisturize, and increase the antioxidant capacity of skin tissue. These findings suggest that puerarin can protect the skin against photoaging.
Keywords: anti-photoaging; antioxidant; oxidative stress; puerarin.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Kato S., Matsui H., Saitoh Y., Miwa N. Fish collagen-containing drink is subcutaneously absorbed and attenuates the UVA-induced tissue-integrity destruction and DNA damages in 3D-human skin tissue model. J. Funct. Foods. 2011;3:50–55. doi: 10.1016/j.jff.2010.11.005. - DOI
-
- Gu Z.P., Lian W.Y., Chen B.Z., Chen S.B., Wang S.B. Investigation on the resources of Puerariae. J. Chin. Med. Mater. 1993;08:13–14. doi: 10.13863/j.issn1001-4454.1993.08.007. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

