Transepithelial Effect of Probiotics in a Novel Model of Gut Lumen to Nerve Signaling
- PMID: 36432542
- PMCID: PMC9697698
- DOI: 10.3390/nu14224856
Transepithelial Effect of Probiotics in a Novel Model of Gut Lumen to Nerve Signaling
Abstract
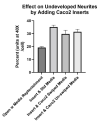
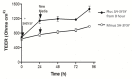
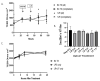
Recent studies have shown that the gut microbiome changes brain function, behavior, and psychiatric and neurological disorders. The Gut-Brain Axis (GBA) provides a neuronal pathway to explain this. But exactly how do commensal bacteria signal through the epithelial layer of the large intestine to activate GBA nerve afferents? An in vitro model is described. We differentiated two human cell lines: Caco2Bbe1 into mature epithelium on 0.4-micron filters and then SH-SY5Y into mature neurons in 24-well plates. These were co-cultured by placing the epithelium-laden filters 1 mm above the neurons. Twenty-four hours later they were tri-cultured by apical addition of 107Lactobacillus rhamnosus or Lactobacillus fermentum which settled on the epithelium. Alone, the Caco2bbe1 cells stimulated neurite outgrowth in underlying SH-SY5Y. Beyond this, the lactobacilli were well tolerated and stimulated further neurite outgrowth by 24 h post-treatment, though not passing through the filters. The results provide face validity for a first-of-kind model of transepithelial intestinal lumen-to nerve signaling. The model displays the tight junctional barrier characteristics found in the large intestine while at the same time translating stimulatory signals from the bacteria through epithelial cells to attracted neurons. The model is easy to set-up with components widely available.
Keywords: Caco2; SH-SY5Y; brain; cholinergic; commensal; enteric; gut; gut brain axis; nervous system; neurites; probiotics; transwell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

