Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer
- PMID: 36432618
- PMCID: PMC9694619
- DOI: 10.3390/nu14224932
Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer
Abstract
The ketone bodies (KBs) β-hydroxybutyrate and acetoacetate are important alternative energy sources for glucose during nutrient deprivation. KBs synthesized by hepatic ketogenesis are catabolized to acetyl-CoA through ketolysis in extrahepatic tissues, followed by the tricarboxylic acid cycle and electron transport chain for ATP production. Ketogenesis and ketolysis are regulated by the key rate-limiting enzymes, 3-hydroxy-3-methylglutaryl-CoA synthase 2 and succinyl-CoA:3-oxoacid-CoA transferase, respectively. KBs participate in various cellular processes as signaling molecules. KBs bind to G protein-coupled receptors. The most abundant KB, β-hydroxybutyrate, regulates gene expression and other cellular functions by inducing post-translational modifications. KBs protect tissues by regulating inflammation and oxidative stress. Recently, interest in KBs has been increasing due to their potential for treatment of various diseases such as neurological and cardiovascular diseases and cancer. Cancer cells reprogram their metabolism to maintain rapid cell growth and proliferation. Dysregulation of KB metabolism also plays a role in tumorigenesis in various types of cancer. Targeting metabolic changes through dietary interventions, including fasting and ketogenic diets, has shown beneficial effects in cancer therapy. Here, we review current knowledge of the molecular mechanisms involved in the regulation of KB metabolism and cellular signaling functions, and the therapeutic potential of KBs and ketogenic diets in cancer.
Keywords: cancer; inflammation; ketogenic diet; ketone bodies; oxidative stress; post-translational modifications; β-hydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
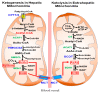
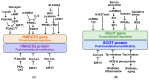
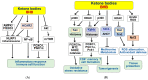
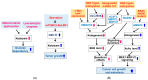
 ) and red (
) and red ( ) arrows indicate increases and decreases, respectively.
) arrows indicate increases and decreases, respectively.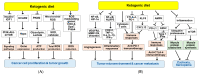
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

