Determination of Metabolomics Profiling in BPA-Induced Impaired Metabolism
- PMID: 36432690
- PMCID: PMC9692868
- DOI: 10.3390/pharmaceutics14112496
Determination of Metabolomics Profiling in BPA-Induced Impaired Metabolism
Abstract
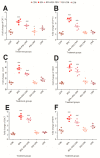
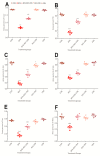
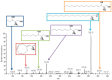
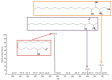
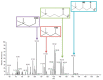
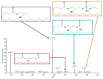
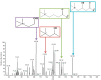
Exposure to bisphenol A (BPA) is unavoidable and it has far-reaching negative effects on living systems. This study aimed to explore the toxic effects of BPA in an experimental animal model through a metabolomics approach that is useful in measuring small molecule perturbations. Beside this, we also examined the ameliorative effects of resveratrol (RSV) against BPA-induced disturbances in experimental mice. This study was conducted for 28 days, and the results showed that BPA indeed induced an impairment in amino acid metabolism, taking place in the mitochondria by significantly (p < 0.05) decreasing the levels of certain amino acids, i.e., taurine, threonine, asparagine, leucine, norleucine, and glutamic acid in the mice plasma. However, the administration of RSV did prove effective against the BPA-induced intoxication and significantly (p < 0.05) restored the level of free amino acids. Lipid metabolites, L-carnitine, sphinganine, phytosphingosine, and lysophosphatidylcholine were also determined in the mice serum. A significant (p < 0.05) decline in glutathione peroxidase (GPx), superoxide dismutase (SOD,) glutathione, and catalase levels and an elevation in malondialdehyde level in the BPA group confirmed the generation of oxidative stress and lipid peroxidation in experimental mice exposed to BPA. The expression of Carnitine palmitoyltransferase I (CPT-I), carnitine palmitoyltransferase II (CPT-II), lecithin−cholesterol acyltransferase (LCAT), carnitine O-octanoyltransferase (CROT), carnitine-acylcarnitine translocase (CACT), and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) genes was significantly upregulated in the liver tissue homogenates of experimental mice exposed to BPA, although RSV regulated the expression of these genes when compared with BPA treated experimental mice. CPT-I, CPT-II, and CACT genes are located in the mitochondria and are involved in the metabolism and transportation of carnitine. Hence, this study confirms that BPA exposure induced oxidative stress, upregulated gene expression, and impaired lipid and amino acid metabolism in experimental mice.
Keywords: amino acid metabolism; gene expression; lipid metabolism; mitochondria; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Oldring P.K., Castle L., O’Mahony C., Dixon J. Estimates of dietary exposure to bisphenol A (BPA) from light metal packaging using food consumption and packaging usage data: A refined deterministic approach and a fully probabilistic (FACET) approach. Food Addit. Contaminants. Part A Chem. Anal. Control. Expo. Risk Assess. 2014;31:466–489. doi: 10.1080/19440049.2013.860240. - DOI - PMC - PubMed
-
- Akash M.S.H., Ejaz ul Haq M., Sharif H., Rehman K. Bisphenol A and Neurological Disorders: From Exposure to Preventive Interventions. In: Akash M.S.H., Rehman K., editors. Environmental Contaminants and Neurological Disorders. Springer International Publishing; Cham, Switzerland: 2021. pp. 185–200. - DOI
-
- Irshad K., Rehman K., Sharif H., Tariq M., Murtaza G., Ibrahim M., Akash M.S.H. Bisphenol A as an EDC in Metabolic Disorders. In: Akash M.S.H., Rehman K., Hashmi M.Z., editors. Endocrine Disrupting Chemicals-Induced Metabolic Disorders and Treatment Strategies. Springer International Publishing; Cham, Switzerland: 2021. pp. 251–263. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

