Repurposing the Antibacterial Agents Peptide 19-4LF and Peptide 19-2.5 for Treatment of Cutaneous Leishmaniasis
- PMID: 36432719
- PMCID: PMC9697117
- DOI: 10.3390/pharmaceutics14112528
Repurposing the Antibacterial Agents Peptide 19-4LF and Peptide 19-2.5 for Treatment of Cutaneous Leishmaniasis
Abstract
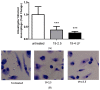
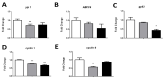
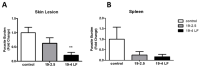
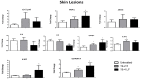
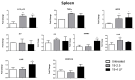
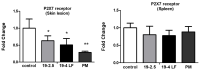
The lack of safe and cost-effective treatments against leishmaniasis highlights the urgent need to develop improved leishmanicidal agents. Antimicrobial peptides (AMPs) are an emerging category of therapeutics exerting a wide range of biological activities such as anti-bacterial, anti-fungal, anti-parasitic and anti-tumoral. In the present study, the approach of repurposing AMPs as antileishmanial drugs was applied. The leishmanicidal activity of two synthetic anti-lipopolysaccharide peptides (SALPs), so-called 19-2.5 and 19-4LF was characterized in Leishmania major. In vitro, both peptides were highly active against intracellular Leishmania major in mouse macrophages without exerting toxicity in host cells. Then, q-PCR-based gene profiling, revealed that this activity was related to the downregulation of several genes involved in drug resistance (yip1), virulence (gp63) and parasite proliferation (Cyclin 1 and Cyclin 6). Importantly, the treatment of BALB/c mice with any of the two AMPs caused a significant reduction in L. major infective burden. This effect was associated with an increase in Th1 cytokine levels (IL-12p35, TNF-α, and iNOS) in the skin lesion and spleen of the L. major infected mice while the Th2-associated genes were downregulated (IL-4 and IL-6). Lastly, we investigated the effect of both peptides in the gene expression profile of the P2X7 purinergic receptor, which has been reported as a therapeutic target in several diseases. The results showed significant repression of P2X7R by both peptides in the skin lesion of L. major infected mice to an extent comparable to that of a common anti-leishmanial drug, Paromomycin. Our in vitro and in vivo studies suggest that the synthetic AMPs 19-2.5 and 19-4LF are promising candidates for leishmaniasis treatment and present P2X7R as a potential therapeutic target in cutaneous leishmaniasis (CL).
Keywords: antimicrobial peptides (AMPs); cytokines; drug repurposing; drug resistance; leishmaniasis; peptide 19-2.5; peptide 19-4LF; proliferation.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae.Acta Biochim Biophys Sin (Shanghai). 2019 Dec 13;51(12):1286-1292. doi: 10.1093/abbs/gmz128. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31761925
-
Combination of paromomycin plus human anti-TNF-α antibodies to control the local inflammatory response in BALB/ mice with cutaneous leishmaniasis lesions.J Dermatol Sci. 2018 Oct;92(1):78-88. doi: 10.1016/j.jdermsci.2018.07.005. Epub 2018 Jul 17. J Dermatol Sci. 2018. PMID: 30037731
-
The potential therapeutic effect of adipose-derived mesenchymal stem cells in the treatment of cutaneous leishmaniasis caused by L. major in BALB/c mice.Exp Parasitol. 2021 Mar;222:108063. doi: 10.1016/j.exppara.2020.108063. Epub 2021 Jan 4. Exp Parasitol. 2021. PMID: 33412170
-
Activity of Anti-Microbial Peptides (AMPs) against Leishmania and Other Parasites: An Overview.Biomolecules. 2021 Jul 4;11(7):984. doi: 10.3390/biom11070984. Biomolecules. 2021. PMID: 34356608 Free PMC article. Review.
-
Prospects for antimicrobial peptide-based immunotherapy approaches in Leishmania control.Expert Rev Anti Infect Ther. 2018 Jun;16(6):461-469. doi: 10.1080/14787210.2018.1483720. Epub 2018 Jun 12. Expert Rev Anti Infect Ther. 2018. PMID: 29889579 Review.
Cited by
-
In Vitro and In Vivo Antileishmanial Activity of Thioridazine.Acta Parasitol. 2024 Mar;69(1):324-331. doi: 10.1007/s11686-023-00746-2. Epub 2023 Dec 9. Acta Parasitol. 2024. PMID: 38070122 Free PMC article.
-
Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease.Pathogens. 2023 Jul 15;12(7):939. doi: 10.3390/pathogens12070939. Pathogens. 2023. PMID: 37513786 Free PMC article. Review.
-
Promising aryl selenoate derivatives as antileishmanial agents and their effects on gene expression.Antimicrob Agents Chemother. 2024 Apr 3;68(4):e0155923. doi: 10.1128/aac.01559-23. Epub 2024 Mar 18. Antimicrob Agents Chemother. 2024. PMID: 38497616 Free PMC article.
References
-
- Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A., et al. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. Erratum in Lancet 2017, 390, e38. - DOI - PMC - PubMed
-
- WHO Expert Committee on the Control of the Leishmaniases and World Health Organization Control of the leishmaniases; Proceedings of the WHO Expert Commitee on the Control of Leishmaniases; Geneva, Switzerland. 22–26 March 2010.

