Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate
- PMID: 36433162
- PMCID: PMC9698909
- DOI: 10.3390/polym14225035
Pyrolysis of Denim Jeans Waste: Pyrolytic Product Modification by the Addition of Sodium Carbonate
Abstract
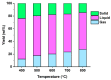
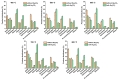
Quickly changing fashion trends generate tremendous amounts of textile waste globally. The inhomogeneity and complicated nature of textile waste make its recycling challenging. Hence, it is urgent to develop a feasible method to extract value from textile waste. Pyrolysis is an effective waste-to-energy option to processing waste feedstocks having an inhomogeneous and complicated nature. Herein, pyrolysis of denim jeans waste (DJW; a textile waste surrogate) was performed in a continuous flow pyrolyser. The effects of adding sodium carbonate (Na2CO3; feedstock/Na2CO3 = 10, weight basis) to the DJW pyrolysis on the yield and composition of pyrolysates were explored. For the DJW pyrolysis, using Na2CO3 as an additive increased the yields of gas and solid phase pyrolysates and decreased the yield of liquid phase pyrolysate. The highest yield of the gas phase pyrolysate was 34.1 wt% at 800 °C in the presence of Na2CO3. The addition of Na2CO3 could increase the contents of combustible gases such as H2 and CO in the gas phase pyrolysate in comparison with the DJW pyrolysis without Na2CO3. The maximum yield of the liquid phase pyrolysate obtained with Na2CO3 was 62.5 wt% at 400 °C. The composition of the liquid phase pyrolysate indicated that the Na2CO3 additive decreased the contents of organic acids, which potentially improve its fuel property by reducing acid value. The results indicated that Na2CO3 can be a potential additive to pyrolysis to enhance energy recovery from DJW.
Keywords: synthetic fiber; thermochemical conversion process; waste recycling; waste treatment; waste-to-energy.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have influenced the work reported in this paper.
Figures



Similar articles
-
Upcycling textile waste using pyrolysis process.Sci Total Environ. 2023 Feb 10;859(Pt 2):160393. doi: 10.1016/j.scitotenv.2022.160393. Epub 2022 Nov 22. Sci Total Environ. 2023. PMID: 36423842
-
Pyrolysis for Nylon 6 Monomer Recovery from Teabag Waste.Polymers (Basel). 2020 Nov 16;12(11):2695. doi: 10.3390/polym12112695. Polymers (Basel). 2020. PMID: 33207591 Free PMC article.
-
Single-Use Disposable Waste Upcycling via Thermochemical Conversion Pathway.Polymers (Basel). 2021 Aug 6;13(16):2617. doi: 10.3390/polym13162617. Polymers (Basel). 2021. PMID: 34451157 Free PMC article.
-
Co-pyrolysis of food waste and wood bark to produce hydrogen with minimizing pollutant emissions.Environ Pollut. 2021 Feb 1;270:116045. doi: 10.1016/j.envpol.2020.116045. Epub 2020 Nov 21. Environ Pollut. 2021. PMID: 33257148
-
Post-consumer textile thermochemical recycling to fuels and biocarbon: A critical review.Sci Total Environ. 2022 Aug 15;834:155387. doi: 10.1016/j.scitotenv.2022.155387. Epub 2022 Apr 21. Sci Total Environ. 2022. PMID: 35461931 Review.
References
-
- US EPA . Facts and Figures about Materials, Waste and Recycling—Textiles: Material-Specific Data. United States Environmental Protection Agency (US EPA); Washington, DC, USA: 2020.
-
- Gupta R., Shukla V.K., Agarwal P. Sustainable transformation in modest fashion through “RPET technology” and “Dry-Dye” process, using recycled PET plastic. Int. J. Recent Technol. Eng. 2019;8:A1432058119. doi: 10.35940/ijrte.A1432.098319. - DOI
-
- EEA . Plastic in Textiles: Towards a Circular Economy for Synthetic Textiles in Europe. European Environment Agency (EEA); Copenhagen, Denmark: 2021.
-
- Lee J., Kwon E.E., Lam S.S., Chen W.-H., Rinklebe J., Park Y.-K. Chemical recycling of plastic waste via thermocatalytic routes. J. Clean. Prod. 2021;321:128989. doi: 10.1016/j.jclepro.2021.128989. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

