Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review
- PMID: 36433257
- PMCID: PMC9697565
- DOI: 10.3390/s22228661
Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review
Abstract
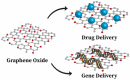
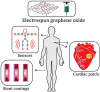
The extraordinary material graphene arrived in the fields of engineering and science to instigate a material revolution in 2004. Graphene has promptly risen as the super star due to its outstanding properties. Graphene is an allotrope of carbon and is made up of sp2-bonded carbon atoms placed in a two-dimensional honeycomb lattice. Graphite consists of stacked layers of graphene. Due to the distinctive structural features as well as excellent physico-chemical and electrical conductivity, graphene allows remarkable improvement in the performance of electrospun nanofibers (NFs), which results in the enhancement of promising applications in NF-based sensor and biomedical technologies. Electrospinning is an easy, economical, and versatile technology depending on electrostatic repulsion between the surface charges to generate fibers from the extensive list of polymeric and ceramic materials with diameters down to a few nanometers. NFs have emerged as important and attractive platform with outstanding properties for biosensing and biomedical applications, because of their excellent functional features, that include high porosity, high surface area to volume ratio, high catalytic and charge transfer, much better electrical conductivity, controllable nanofiber mat configuration, biocompatibility, and bioresorbability. The inclusion of graphene nanomaterials (GNMs) into NFs is highly desirable. Pre-processing techniques and post-processing techniques to incorporate GNMs into electrospun polymer NFs are precisely discussed. The accomplishment and the utilization of NFs containing GNMs in the electrochemical biosensing pathway for the detection of a broad range biological analytes are discussed. Graphene oxide (GO) has great importance and potential in the biomedical field and can imitate the composition of the extracellular matrix. The oxygen-rich GO is hydrophilic in nature and easily disperses in water, and assists in cell growth, drug delivery, and antimicrobial properties of electrospun nanofiber matrices. NFs containing GO for tissue engineering, drug and gene delivery, wound healing applications, and medical equipment are discussed. NFs containing GO have importance in biomedical applications, which include engineered cardiac patches, instrument coatings, and triboelectric nanogenerators (TENGs) for motion sensing applications. This review deals with graphene-based nanomaterials (GNMs) such as GO incorporated electrospun polymeric NFs for biosensing and biomedical applications, that can bridge the gap between the laboratory facility and industry.
Keywords: biomedical applications; drug delivery; electrochemical biosensors; electrospinning; electrospun nanofibers; graphene; graphene oxide; medical devices; tissue engineering; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

