Vascular smooth muscle cell senescence accelerates medin aggregation via small extracellular vesicle secretion and extracellular matrix reorganization
- PMID: 36433666
- PMCID: PMC9924949
- DOI: 10.1111/acel.13746
Vascular smooth muscle cell senescence accelerates medin aggregation via small extracellular vesicle secretion and extracellular matrix reorganization
Abstract
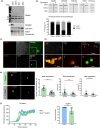
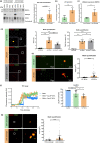
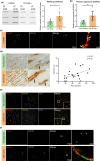
Vascular amyloidosis, caused when peptide monomers aggregate into insoluble amyloid, is a prevalent age-associated pathology. Aortic medial amyloid (AMA) is the most common human amyloid and is composed of medin, a 50-amino acid peptide. Emerging evidence has implicated extracellular vesicles (EVs) as mediators of pathological amyloid accumulation in the extracellular matrix (ECM). To determine the mechanisms of AMA formation with age, we explored the impact of vascular smooth muscle cell (VSMC) senescence, EV secretion, and ECM remodeling on medin accumulation. Medin was detected in EVs secreted from primary VSMCs. Small, round medin aggregates colocalized with EV markers in decellularized ECM in vitro and medin was shown on the surface of EVs deposited in the ECM. Decreasing EV secretion with an inhibitor attenuated aggregation and deposition of medin in the ECM. Medin accumulation in the aortic wall of human subjects was strongly correlated with age and VSMC senescence increased EV secretion, increased EV medin loading and triggered deposition of fibril-like medin. Proteomic analysis showed VSMC senescence induced changes in EV cargo and ECM composition, which led to enhanced EV-ECM binding and accelerated medin aggregation. Abundance of the proteoglycan, HSPG2, was increased in the senescent ECM and colocalized with EVs and medin. Isolated EVs selectively bound to HSPG2 in the ECM and its knock-down decreased formation of fibril-like medin structures. These data identify VSMC-derived EVs and HSPG2 in the ECM as key mediators of medin accumulation, contributing to age-associated AMA development.
Keywords: amyloid; extracellular matrix; extracellular vesicles; proteoglycans.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness.Circ Res. 2024 Feb 2;134(3):307-324. doi: 10.1161/CIRCRESAHA.123.323365. Epub 2024 Jan 5. Circ Res. 2024. PMID: 38179698 Free PMC article.
-
Gla Rich Protein (GRP) Mediates Vascular Smooth Muscle Cell (VSMC) Osteogenic Differentiation, Extracellular Vesicle (EV) Calcification Propensity, and Immunomodulatory Properties.Int J Mol Sci. 2024 Nov 19;25(22):12406. doi: 10.3390/ijms252212406. Int J Mol Sci. 2024. PMID: 39596469 Free PMC article.
-
Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions.Geroscience. 2022 Dec;44(6):2863-2884. doi: 10.1007/s11357-022-00625-0. Epub 2022 Jul 28. Geroscience. 2022. PMID: 35900662 Free PMC article.
-
Direct and cell-mediated EV-ECM interplay.Acta Biomater. 2024 Sep 15;186:63-84. doi: 10.1016/j.actbio.2024.07.029. Epub 2024 Jul 21. Acta Biomater. 2024. PMID: 39043290 Review.
-
Matrix Metalloproteinase 2 as a Potential Mediator of Vascular Smooth Muscle Cell Migration and Chronic Vascular Remodeling in Hypertension.J Vasc Res. 2015;52(4):221-31. doi: 10.1159/000441621. Epub 2016 Jan 6. J Vasc Res. 2015. PMID: 26731549 Review.
Cited by
-
Aortic Stiffness and Alzheimer's Disease: The Medin Connection.Biomolecules. 2025 Aug 8;15(8):1148. doi: 10.3390/biom15081148. Biomolecules. 2025. PMID: 40867593 Free PMC article. Review.
-
Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration.Int J Mol Sci. 2025 May 19;26(10):4852. doi: 10.3390/ijms26104852. Int J Mol Sci. 2025. PMID: 40429992 Free PMC article.
-
Vascular Aging and Atherosclerosis: A Perspective on Aging.Aging Dis. 2024 Mar 6;16(1):33-48. doi: 10.14336/AD.2024.0201-1. Online ahead of print. Aging Dis. 2024. PMID: 38502584 Free PMC article.
-
Extracellular matrix in vascular homeostasis and disease.Nat Rev Cardiol. 2025 May;22(5):333-353. doi: 10.1038/s41569-024-01103-0. Epub 2025 Jan 2. Nat Rev Cardiol. 2025. PMID: 39743560 Review.
-
Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness.Circ Res. 2024 Feb 2;134(3):307-324. doi: 10.1161/CIRCRESAHA.123.323365. Epub 2024 Jan 5. Circ Res. 2024. PMID: 38179698 Free PMC article.
References
-
- Acosta, J. C. , Banito, A. , Wuestefeld, T. , Georgilis, A. , Janich, P. , Morton, J. P. , Athineos, D. , Kang, T. W. , Lasitschka, F. , Andrulis, M. , Pascual, G. , Morris, K. J. , Khan, S. , Jin, H. , Dharmalingam, G. , Snijders, A. P. , Carroll, T. , Capper, D. , Pritchard, C. , … Gil, J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology, 15(8), 978–990. 10.1038/ncb2784 - DOI - PMC - PubMed
-
- Alvarez‐Martinez, M. T. , Fontes, P. , Zomosa‐Signoret, V. , Arnaud, J. D. , Hingant, E. , Pujo‐Menjouet, L. , & Liautard, J. P. (2011). Dynamics of polymerization shed light on the mechanisms that lead to multiple amyloid structures of the prion protein. Biochimica et Biophysica Acta, 1814(10), 1305–1317. 10.1016/j.bbapap.2011.05.016 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

