Elevated granulocyte colony stimulating factor (CSF) causes cerebellar deficits and anxiety in a model of CSF-1 receptor related leukodystrophy
- PMID: 36433736
- PMCID: PMC9868112
- DOI: 10.1002/glia.24310
Elevated granulocyte colony stimulating factor (CSF) causes cerebellar deficits and anxiety in a model of CSF-1 receptor related leukodystrophy
Abstract
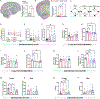
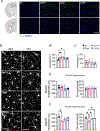
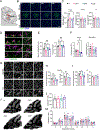
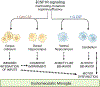
Colony stimulating factor (CSF) receptor-1 (CSF-1R)-related leukoencephalopathy (CRL) is an adult-onset, demyelinating and neurodegenerative disease caused by autosomal dominant mutations in CSF1R, modeled by the Csf1r+/- mouse. The expression of Csf2, encoding granulocyte-macrophage CSF (GM-CSF) and of Csf3, encoding granulocyte CSF (G-CSF), are elevated in both mouse and human CRL brains. While monoallelic targeting of Csf2 has been shown to attenuate many behavioral and histological deficits of Csf1r+/- mice, including cognitive dysfunction and demyelination, the contribution of Csf3 has not been explored. In the present study, we investigate the behavioral, electrophysiological and histopathological phenotypes of Csf1r+/- mice following monoallelic targeting of Csf3. We show that Csf3 heterozygosity normalized the Csf3 levels in Csf1r+/- mouse brains and ameliorated anxiety-like behavior, motor coordination and social interaction deficits, but not the cognitive impairment of Csf1r+/- mice. Csf3 heterozygosity failed to prevent callosal demyelination. However, consistent with its effects on behavior, Csf3 heterozygosity normalized microglial morphology in the cerebellum and in the ventral, but not in the dorsal hippocampus. Csf1r+/- mice exhibited altered firing activity in the deep cerebellar nuclei (DCN) associated with increased engulfment of glutamatergic synapses by DCN microglia and increased deposition of the complement factor C1q on glutamatergic synapses. These phenotypes were significantly ameliorated by monoallelic deletion of Csf3. Our current and earlier findings indicate that G-CSF and GM-CSF play largely non-overlapping roles in CRL-like disease development in Csf1r+/- mice.
Keywords: CRL; CSF-1 receptor; G-CSF; HDLS; cerebellum; leukoencephalopathy; microglia.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Figures



Similar articles
-
Microglial reduction of colony stimulating factor-1 receptor expression is sufficient to confer adult onset leukodystrophy.Glia. 2021 Mar;69(3):779-791. doi: 10.1002/glia.23929. Epub 2020 Oct 20. Glia. 2021. PMID: 33079443 Free PMC article.
-
Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP).Neurobiol Dis. 2015 Feb;74:219-28. doi: 10.1016/j.nbd.2014.12.001. Epub 2014 Dec 9. Neurobiol Dis. 2015. PMID: 25497733 Free PMC article.
-
Microglial Homeostasis Requires Balanced CSF-1/CSF-2 Receptor Signaling.Cell Rep. 2020 Mar 3;30(9):3004-3019.e5. doi: 10.1016/j.celrep.2020.02.028. Cell Rep. 2020. PMID: 32130903 Free PMC article.
-
Modeling CSF-1 receptor deficiency diseases - how close are we?FEBS J. 2022 Sep;289(17):5049-5073. doi: 10.1111/febs.16085. Epub 2021 Jul 5. FEBS J. 2022. PMID: 34145972 Free PMC article. Review.
-
Regulation of myeloid development and function by colony stimulating factors.Dev Comp Immunol. 2004 May 3;28(5):509-54. doi: 10.1016/j.dci.2003.09.010. Dev Comp Immunol. 2004. PMID: 15062647 Review.
Cited by
-
Prophylactic effect of chronic immunosuppression in a mouse model of CSF-1 receptor-related leukoencephalopathy.Glia. 2023 Nov;71(11):2664-2678. doi: 10.1002/glia.24446. Epub 2023 Jul 30. Glia. 2023. PMID: 37519044 Free PMC article.
-
Differential regulation of microglial states by colony stimulating factors.Front Cell Neurosci. 2023 Oct 30;17:1275935. doi: 10.3389/fncel.2023.1275935. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37964794 Free PMC article. Review.
-
Identification of potential biomarkers and therapeutic targets for antineutrophil cytoplasmic antibody-associated glomerulonephritis.iScience. 2023 Oct 7;26(11):108157. doi: 10.1016/j.isci.2023.108157. eCollection 2023 Nov 17. iScience. 2023. PMID: 37915598 Free PMC article.
-
Cerebellar microglia: On the edge between neuroinflammation and neuroregulation.Neural Regen Res. 2026 Jan 1;21(1):156-172. doi: 10.4103/NRR.NRR-D-24-00550. Epub 2024 Oct 22. Neural Regen Res. 2026. PMID: 40489344 Free PMC article.
References
-
- Amirzagar N, Nafissi S, Tafakhori A, Modabbernia A, Amirzargar A, Ghaffarpour M, … Harirchian MH. (2015). Granulocyte colony-stimulating factor for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled study of Iranian patients. J Clin Neurol, 11(2), 164–171. doi:10.3988/jcn.2015.11.2.164 - DOI - PMC - PubMed
-
- Arreola MA, Soni N, Crapser JD, Hohsfield LA, Elmore MRP, Matheos DP, … Green KN. (2021). Microglial dyshomeostasis drives perineuronal net and synaptic loss in a CSF1R(+/−) mouse model of ALSP, which can be rescued via CSF1R inhibitors. Sci Adv, 7(35). doi:10.1126/sciadv.abg1601 - DOI - PMC - PubMed
-
- Basso L, Lapointe TK, Iftinca M, Marsters C, Hollenberg MD, Kurrasch DM, & Altier C (2017). Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis. Proc Natl Acad Sci U S A, 114(42), 11235–11240. doi:10.1073/pnas.1706053114 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- U19AG063911/NH/NIH HHS/United States
- P30CA013330/NH/NIH HHS/United States
- U54 HD090260/NH/NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- U54 HD090260/HD/NICHD NIH HHS/United States
- P50 HD105352/HD/NICHD NIH HHS/United States
- R01NS091519/NH/NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- R21MH114182/NH/NIH HHS/United States
- R01 NS105470/NS/NINDS NIH HHS/United States
- R01 NS091519/NS/NINDS NIH HHS/United States
- R01NS105470/NH/NIH HHS/United States
- 1U19AG063911/NH/NIH HHS/United States
- R21 MH114182/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

