CXCL13 is a predictive biomarker in idiopathic multicentric Castleman disease
- PMID: 36433996
- PMCID: PMC9700691
- DOI: 10.1038/s41467-022-34873-7
CXCL13 is a predictive biomarker in idiopathic multicentric Castleman disease
Abstract
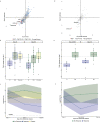
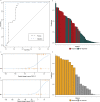
Idiopathic multicentric Castleman disease (iMCD) is a rare and poorly-understood cytokine storm-driven inflammatory disorder. Interleukin-6 (IL-6) is a known disease driver in some patients, but anti-IL-6 therapy with siltuximab is not effective in all patients, and biomarkers indicating success at an early time point following treatment initiation are lacking. Here we show, by comparison of levels of 1,178 proteins in sera of healthy participants (N = 42), patients with iMCD (N = 88), and with related diseases (N = 60), a comprehensive landscape of candidate disease mediators and predictors of siltuximab response. C-X-C Motif Chemokine Ligand-13 (CXCL13) is identified and validated as the protein most prominently up-regulated in iMCD. Early and significant decrease in CXCL13 levels clearly distinguishes siltuximab responders from non-responders; a 17% reduction by day 8 following siltuximab therapy initiation is predictive of response at later time points. Our study thus suggests that CXCL13 is a predictive biomarker of response to siltuximab in iMCD.
© 2022. The Author(s).
Conflict of interest statement
D.C.F. has received research funding for the ACCELERATE registry from EUSA Pharma and consulting fees from EUSA Pharma, and Pfizer provides a study drug with no associated research funding for the clinical trial of sirolimus (NCT03933904). D.C.F. has two provisional patents pending related to the diagnosis and treatment of iMCD, including one related to CXCL13 as a biomarker in iMCD. A.B.O., L.K., and P.B. are employed by and/or receive equity ownership from Medidata Solutions, a subsidiary of 3DS. A.F. has received honoraria and research support from Janssen Pharmaceuticals and EUSA Pharma. F.v.R. provides consultancy for EUSA Pharma, Takeda, Sanofi Genzyme, Adicet Bio, Kite Pharma, and Karyopharm Therapeutics. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

