A non-carboxylating pentose bisphosphate pathway in halophilic archaea
- PMID: 36434094
- PMCID: PMC9700705
- DOI: 10.1038/s42003-022-04247-2
A non-carboxylating pentose bisphosphate pathway in halophilic archaea
Abstract
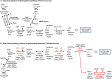
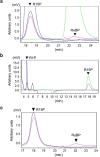
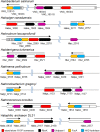
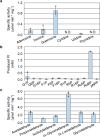
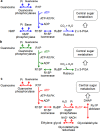
Bacteria and Eucarya utilize the non-oxidative pentose phosphate pathway to direct the ribose moieties of nucleosides to central carbon metabolism. Many archaea do not possess this pathway, and instead, Thermococcales utilize a pentose bisphosphate pathway involving ribose-1,5-bisphosphate (R15P) isomerase and ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco). Intriguingly, multiple genomes from halophilic archaea seem only to harbor R15P isomerase, and do not harbor Rubisco. In this study, we identify a previously unrecognized nucleoside degradation pathway in halophilic archaea, composed of guanosine phosphorylase, ATP-dependent ribose-1-phosphate kinase, R15P isomerase, RuBP phosphatase, ribulose-1-phosphate aldolase, and glycolaldehyde reductase. The pathway converts the ribose moiety of guanosine to dihydroxyacetone phosphate and ethylene glycol. Although the metabolic route from guanosine to RuBP via R15P is similar to that of the pentose bisphosphate pathway in Thermococcales, the downstream route does not utilize Rubisco and is unique to halophilic archaea.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

