Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: role in sarcopenia
- PMID: 36434267
- PMCID: PMC9700804
- DOI: 10.1038/s42003-022-04246-3
Vitamin D status modulates mitochondrial oxidative capacities in skeletal muscle: role in sarcopenia
Abstract
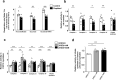
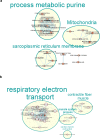
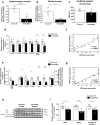
Skeletal muscle mitochondrial function is the biggest component of whole-body energy output. Mitochondrial energy production during exercise is impaired in vitamin D-deficient subjects. In cultured myotubes, loss of vitamin D receptor (VDR) function decreases mitochondrial respiration rate and ATP production from oxidative phosphorylation. We aimed to examine the effects of vitamin D deficiency and supplementation on whole-body energy expenditure and muscle mitochondrial function in old rats, old mice, and human subjects. To gain further insight into the mechanisms involved, we used C2C12 and human muscle cells and transgenic mice with muscle-specific VDR tamoxifen-inducible deficiency. We observed that in vivo and in vitro vitamin D fluctuations changed mitochondrial biogenesis and oxidative activity in skeletal muscle. Vitamin D supplementation initiated in older people improved muscle mass and strength. We hypothesize that vitamin D supplementation is likely to help prevent not only sarcopenia but also sarcopenic obesity in vitamin D-deficient subjects.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: this study was partly supported by a grant from Danone Nutricia Research, Utrecht, The Netherlands, and, M.J., Y.L., M.F., and M.V.D. are employees of Danone Nutricia Research, Utrecht, The Netherlands.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

