Therapeutic Hypothermia Combined with Hydrogen Sulfide Treatment Attenuated Early Blood-Brain Barrier Disruption and Brain Edema Induced by Cardiac Arrest and Resuscitation in Rat Model
- PMID: 36434369
- PMCID: PMC9922226
- DOI: 10.1007/s11064-022-03824-5
Therapeutic Hypothermia Combined with Hydrogen Sulfide Treatment Attenuated Early Blood-Brain Barrier Disruption and Brain Edema Induced by Cardiac Arrest and Resuscitation in Rat Model
Abstract
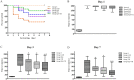
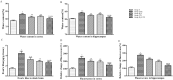
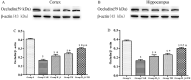
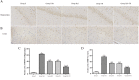
Brain injury remains a major problem in patients suffering cardiac arrest (CA). Disruption of the blood-brain barrier (BBB) is an important factor leading to brain injury. Therapeutic hypothermia is widely accepted to limit neurological impairment. However, the efficacy is incomplete. Hydrogen sulfide (H2S), a signaling gas molecule, has protective effects after cerebral ischemia reperfusion injury. This study showed that combination of hypothermia and H2S after resuscitation was more beneficial for attenuated BBB disruption and brain edema than that of hypothermia or H2S treatment alone. CA was induced by ventricular fibrillation for 4 min. Hypothermia was performed by applying alcohol and ice bags to the body surface under anesthesia. We used sodium hydrosulphide (NaHS) as the H2S donor. We found that global brain ischemia induced by CA and cardiopulmonary resuscitation (CPR) resulted in brain edema and BBB disruption; Hypothermia or H2S treatment diminished brain edema, decreased the permeability and preserved the structure of BBB during the early period of CA and resuscitation, and more importantly, improved the neurologic function, increased the 7-day survival rate after resuscitation; the combination of hypothermia and H2S treatment was more beneficial than that of hypothermia or H2S treatment alone. The beneficial effects were associated with the inhibition of matrix metalloproteinase-9 expression, attenuated the degradation of the tight junction protein occludin, and subsequently protected the structure of BBB. These findings suggest that combined use of therapeutic hypothermia and hydrogen sulfide treatment during resuscitation of CA patients could be a potential strategy to improve clinical outcomes and survival rate.
Keywords: Blood–brain barrier; Cardiac arrest; Cardiopulmonary resuscitation; Hydrogen sulfide; Therapeutic hypothermia.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Hydrogen sulfide inhalation decreases early blood-brain barrier permeability and brain edema induced by cardiac arrest and resuscitation.J Cereb Blood Flow Metab. 2015 Mar;35(3):494-500. doi: 10.1038/jcbfm.2014.223. Epub 2014 Dec 10. J Cereb Blood Flow Metab. 2015. PMID: 25492119 Free PMC article.
-
Mild hypothermia alleviates brain oedema and blood-brain barrier disruption by attenuating tight junction and adherens junction breakdown in a swine model of cardiopulmonary resuscitation.PLoS One. 2017 Mar 29;12(3):e0174596. doi: 10.1371/journal.pone.0174596. eCollection 2017. PLoS One. 2017. PMID: 28355299 Free PMC article.
-
Hydrogen Sulfide Decreases Blood-Brain Barrier Damage via Regulating Protein Kinase C and Tight Junction After Cardiac Arrest in Rats.Cell Physiol Biochem. 2018;47(3):994-1006. doi: 10.1159/000490166. Epub 2018 May 24. Cell Physiol Biochem. 2018. PMID: 29843155
-
Bench to bedside: brain edema and cerebral resuscitation: the present and future.Acad Emerg Med. 2002 Sep;9(9):933-46. doi: 10.1111/j.1553-2712.2002.tb02196.x. Acad Emerg Med. 2002. PMID: 12208684 Review.
-
Cerebral Edema After Cardiopulmonary Resuscitation: A Therapeutic Target Following Cardiac Arrest?Neurocrit Care. 2018 Jun;28(3):276-287. doi: 10.1007/s12028-017-0474-8. Neurocrit Care. 2018. PMID: 29080068 Review.
Cited by
-
Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice.Biomedicines. 2024 Dec 18;12(12):2874. doi: 10.3390/biomedicines12122874. Biomedicines. 2024. PMID: 39767780 Free PMC article.
-
Translational approach to assess brain injury after cardiac arrest in preclinical models: a narrative review.Intensive Care Med Exp. 2025 Jan 14;13(1):3. doi: 10.1186/s40635-024-00710-y. Intensive Care Med Exp. 2025. PMID: 39808393 Free PMC article. Review. No abstract available.
-
The Role of Hydrogen Sulfide in Regulation of Cell Death following Neurotrauma and Related Neurodegenerative and Psychiatric Diseases.Int J Mol Sci. 2023 Jun 28;24(13):10742. doi: 10.3390/ijms241310742. Int J Mol Sci. 2023. PMID: 37445920 Free PMC article. Review.
-
Carnosine alleviates kidney tubular epithelial injury by targeting NRF2 mediated ferroptosis in diabetic nephropathy.Amino Acids. 2023 Sep;55(9):1141-1155. doi: 10.1007/s00726-023-03301-5. Epub 2023 Jul 14. Amino Acids. 2023. PMID: 37450047
-
Application of artificial hibernation technology in acute brain injury.Neural Regen Res. 2024 Sep 1;19(9):1940-1946. doi: 10.4103/1673-5374.390968. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38227519 Free PMC article.
References
-
- Park JS, You Y, Min JH, Yoo I, Jeong W, Cho Y, Ryu S, Lee J, Kim SW, Cho SU, Oh SK, Ahn HJ, Lee J, Lee IH. Study on the timing of severe blood-brain barrier disruption using cerebrospinal fluid-serum albumin quotient in post cardiac arrest patients treated with targeted temperature management. Resuscitation. 2019;135:118–123. doi: 10.1016/j.resuscitation.2018.10.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

