Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: a meta-analysis and meta-regression
- PMID: 36434597
- PMCID: PMC9701077
- DOI: 10.1186/s12916-022-02663-z
Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: a meta-analysis and meta-regression
Abstract
Background: Observational studies made it possible to assess the impact of risk factors on the long-term effectiveness of mRNA and adenoviral vector (AdV) vaccines against COVID-19.
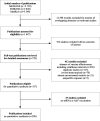
Methods: A computerized literature search was undertaken using the MEDLINE, EMBASE, and MedRxiv databases to identify eligible studies, with no language restrictions, published up to 28 February 2022. Eligible were observational studies assessing vaccine effectiveness (VE) by disease severity with reference groups of unvaccinated participants or participants immunized with one, two, or three vaccine doses. Our study was carried out in compliance with the PRISMA and MOOSE guidelines. The risk of study bias was identified using the Newcastle-Ottawa Quality Assessment Scale. The GRADE guidelines were applied to assess the strength of evidence for the primary outcome. The synthesis was conducted using a meta-analysis and meta-regression.
Results: Out of a total of 14,155 publications, 290 studies were included. Early VE of full vaccination against COVID-19 of any symptomatology and severity decreased from 96% (95% CI, 95-96%) for mRNA and from 86% (95% CI, 83-89%) for AdV vaccines to 67% for both vaccine types in the last 2 months of 2021. A similar 1-year decline from 98 to 86% was found for severe COVID-19 after full immunization with mRNA, but not with AdV vaccines providing persistent 82-87% effectiveness. Variant-reduced VE was only associated with Omicron regardless of disease severity, vaccine type, or vaccination completeness. The level of protection was reduced in participants aged >65 years, with a comorbidity or those in long-term care or residential homes independently of the number of doses received. The booster effect of the third mRNA dose was unclear because incompletely restored effectiveness, regardless of disease severity, declined within a short-term interval of 4 months.
Conclusions: Full vaccination provided an early high, yet waning level of protection against COVID-19 of any severity with a strong impact on the high-risk population. Moreover, the potential risk of new antigenically distinct variants should not be underestimated, and any future immunization strategy should include variant-updated vaccines.
Keywords: Adenoviral vector vaccines; COVID-19; Protection decline; Vaccine effectiveness; mRNA vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Understanding the time-driven shifts of vaccine effectiveness against any and severe COVID-19 before and after the surge of Omicron variants within 2.5 years of vaccination: A meta-regression.Int J Infect Dis. 2024 May;142:106986. doi: 10.1016/j.ijid.2024.106986. Epub 2024 Feb 26. Int J Infect Dis. 2024. PMID: 38417615
-
Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022.MMWR Morb Mortal Wkly Rep. 2022 Jan 21;71(4):139-145. doi: 10.15585/mmwr.mm7104e3. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35085224 Free PMC article.
-
Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022.MMWR Morb Mortal Wkly Rep. 2022 Feb 18;71(7):255-263. doi: 10.15585/mmwr.mm7107e2. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35176007 Free PMC article.
-
The Vaccine Efficacy Against the SARS-CoV-2 Omicron: A Systemic Review and Meta-Analysis.Front Public Health. 2022 Jul 13;10:940956. doi: 10.3389/fpubh.2022.940956. eCollection 2022. Front Public Health. 2022. PMID: 35910897 Free PMC article.
-
SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis.BMC Infect Dis. 2022 May 7;22(1):439. doi: 10.1186/s12879-022-07418-y. BMC Infect Dis. 2022. PMID: 35525973 Free PMC article.
Cited by
-
Anti-SARS-CoV-2 Antibody Testing: Role and Indications.J Clin Med. 2023 Dec 8;12(24):7575. doi: 10.3390/jcm12247575. J Clin Med. 2023. PMID: 38137643 Free PMC article. Review.
-
Individual risk factors associated with SARS-CoV-2 infection during Alpha variant in high-income countries: a systematic review and meta-analysis.Front Public Health. 2024 Jul 30;12:1367480. doi: 10.3389/fpubh.2024.1367480. eCollection 2024. Front Public Health. 2024. PMID: 39139667 Free PMC article.
-
Anti-SARS-CoV-2 Antibodies versus Vaccination Status in CAD Patients with COVID-19: A Prospective, Propensity Score-Matched Cohort Study.Vaccines (Basel). 2024 Jul 30;12(8):855. doi: 10.3390/vaccines12080855. Vaccines (Basel). 2024. PMID: 39203980 Free PMC article.
-
Will Omics Biotechnologies Save Us from Future Pandemics? Lessons from COVID-19 for Vaccinomics and Adversomics.Biomedicines. 2022 Dec 26;11(1):52. doi: 10.3390/biomedicines11010052. Biomedicines. 2022. PMID: 36672560 Free PMC article. Review.
-
Vaccination status and outcomes in critical COVID-19 patients.J Bras Pneumol. 2024 Feb 23;50(1):e20230116. doi: 10.36416/1806-3756/e20230116. eCollection 2024. J Bras Pneumol. 2024. PMID: 38422336 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

