An efficient preparation and biocatalytic synthesis of novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside through using resting cells and macroporous resins
- PMID: 36434691
- PMCID: PMC9700910
- DOI: 10.1186/s13068-022-02228-5
An efficient preparation and biocatalytic synthesis of novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside through using resting cells and macroporous resins
Abstract
Background: C-glycosylated flavonoids are a main type of structural modification and can endow flavonoids with greater stability, bioactivity, and bioavailability. Although some C-glycosylated flavonoids have been biosynthesized in vivo or vitro, only a few C-glycosylflavonols have been prepared by these methods.
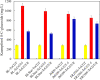
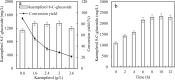
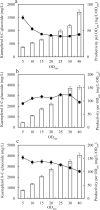
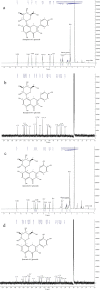
Results: In this study, several uridine 5'-diphosphate (UDP)-glucose biosynthesis pathways and Escherichia coli hosts were screened to reconstruct recombinant strains for producing the novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside. To increase C-glycosylflavonol production, the timing of flavonol addition was adjusted, and glycerol was added to avoid degradation of C-glycosylflavonols. By using resting cell bioconversion, the highest kaempferol 8-C-glucoside and quercetin 8-C-glucoside production reached 16.6 g/L and 12.5 g/L, respectively. Then, ultrasound-assisted adsorption/desorption was used to prepare C-glycosylflavonols by using macroporous resins. Through screening macroporous resins and optimizing the adsorption/desorption conditions, the highest adsorption capacity and desorption capacity for kaempferol 8-C-glucoside on HPD100 reached 28.57 mg/g and 24.15 mg/g, respectively. Finally, kaempferol 8-C-glucoside (15.4 g) with a yield of 93% and quercetin 8-C-glucoside (11.3 g) with a yield of 91% were obtained from 1 L of fermentation broth.
Conclusions: Kaempferol 8-C-glucoside and quercetin 8-C-glucoside are novel C-glycosylflavonols, which have not been extracted from plants. This study provides an efficient method for the preparation and biocatalytic synthesis of kaempferol 8-C-glucoside and quercetin 8-C-glucoside by metabolic engineering of Escherichia coli.
Keywords: Adsorption/desorption; Kaempferol 8-C-glucoside; Metabolic engineering; Quercetin 8-C-glucoside; Resting cell bioconversion; UDP-glucose biosynthesis pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Production of Flavonoid 7-O-glucosides by Bioconversion Using Escherichia coli Expressing a 7-O-glucosyltransferase from Tobacco (Nicotiana tabacum).Appl Biochem Biotechnol. 2022 Jul;194(7):3320-3329. doi: 10.1007/s12010-022-03880-1. Epub 2022 Mar 26. Appl Biochem Biotechnol. 2022. PMID: 35347669
-
Optimizing yeast for high-level production of kaempferol and quercetin.Microb Cell Fact. 2023 Apr 20;22(1):74. doi: 10.1186/s12934-023-02084-4. Microb Cell Fact. 2023. PMID: 37076829 Free PMC article.
-
Flavonoids from Sedum japonicum subsp. oryzifolium (Crassulaceae).Molecules. 2022 Nov 7;27(21):7632. doi: 10.3390/molecules27217632. Molecules. 2022. PMID: 36364459 Free PMC article.
-
Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans.Nutrients. 2019 Sep 25;11(10):2288. doi: 10.3390/nu11102288. Nutrients. 2019. PMID: 31557798 Free PMC article. Review.
-
Quercetin and Related Analogs as Therapeutics to Promote Tissue Repair.Bioengineering (Basel). 2023 Sep 25;10(10):1127. doi: 10.3390/bioengineering10101127. Bioengineering (Basel). 2023. PMID: 37892857 Free PMC article. Review.
Cited by
-
Systematic Engineering of Saccharomyces cerevisiae for the De Novo Biosynthesis of Genistein and Glycosylation Derivatives.J Fungi (Basel). 2024 Feb 26;10(3):176. doi: 10.3390/jof10030176. J Fungi (Basel). 2024. PMID: 38535185 Free PMC article.
-
Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability.Molecules. 2025 Mar 6;30(5):1184. doi: 10.3390/molecules30051184. Molecules. 2025. PMID: 40076406 Free PMC article. Review.
References
-
- Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
