Recombinant human plasma gelsolin reverses increased permeability of the blood-brain barrier induced by the spike protein of the SARS-CoV-2 virus
- PMID: 36434734
- PMCID: PMC9694610
- DOI: 10.1186/s12974-022-02642-4
Recombinant human plasma gelsolin reverses increased permeability of the blood-brain barrier induced by the spike protein of the SARS-CoV-2 virus
Abstract
Background: Plasma gelsolin (pGSN) is an important part of the blood actin buffer that prevents negative consequences of possible F-actin deposition in the microcirculation and has various functions during host immune response. Recent reports reveal that severe COVID-19 correlates with reduced levels of pGSN. Therefore, using an in vitro system, we investigated whether pGSN could attenuate increased permeability of the blood-brain barrier (BBB) during its exposure to the portion of the SARS-CoV-2 spike protein containing the receptor binding domain (S1 subunit).
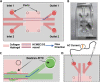
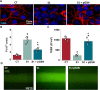
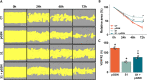
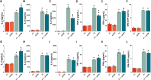
Materials and methods: Two- and three-dimensional models of the human BBB were constructed using the human cerebral microvascular endothelial cell line hCMEC/D3 and exposed to physiologically relevant shear stress to mimic perfusion in the central nervous system (CNS). Trans-endothelial electrical resistance (TEER) as well as immunostaining and Western blotting of tight junction (TJ) proteins assessed barrier integrity in the presence of the SARS-CoV-2 spike protein and pGSN. The IncuCyte Live Imaging system evaluated the motility of the endothelial cells. Magnetic bead-based ELISA was used to determine cytokine secretion. Additionally, quantitative real-time PCR (qRT-PCR) revealed gene expression of proteins from signaling pathways that are associated with the immune response.
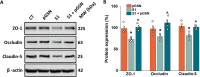
Results: pGSN reversed S1-induced BBB permeability in both 2D and 3D BBB models in the presence of shear stress. BBB models exposed to pGSN also exhibited attenuated pro-inflammatory signaling pathways (PI3K, AKT, MAPK, NF-κB), reduced cytokine secretion (IL-6, IL-8, TNF-α), and increased expression of proteins that form intercellular TJ (ZO-1, occludin, claudin-5).
Conclusion: Due to its anti-inflammatory and protective effects on the brain endothelium, pGSN has the potential to be an alternative therapeutic target for patients with severe SARS-CoV-2 infection, especially those suffering neurological complications of COVID-19.
Keywords: Blood–brain barrier; COVID-19; Microfluidics; Plasma gelsolin (pGSN); SARS-CoV-2; Tissue engineering.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there were no commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier.Neurobiol Dis. 2020 Dec;146:105131. doi: 10.1016/j.nbd.2020.105131. Epub 2020 Oct 11. Neurobiol Dis. 2020. PMID: 33053430 Free PMC article.
-
Longitudinal profiles of plasma gelsolin, cytokines and antibody expression predict COVID-19 severity and hospitalization outcomes.Front Immunol. 2022 Sep 6;13:1011084. doi: 10.3389/fimmu.2022.1011084. eCollection 2022. Front Immunol. 2022. PMID: 36148234 Free PMC article.
-
Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection.J Virol. 2014 May;88(9):4698-710. doi: 10.1128/JVI.03149-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522913 Free PMC article.
-
COVID-19 and Long COVID: Disruption of the Neurovascular Unit, Blood-Brain Barrier, and Tight Junctions.Neuroscientist. 2024 Aug;30(4):421-439. doi: 10.1177/10738584231194927. Epub 2023 Sep 11. Neuroscientist. 2024. PMID: 37694571 Review.
-
Neurological manifestations of SARS-CoV-2: complexity, mechanism and associated disorders.Eur J Med Res. 2023 Aug 30;28(1):307. doi: 10.1186/s40001-023-01293-2. Eur J Med Res. 2023. PMID: 37649125 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Structural Proteins Modulated Blood-Testis Barrier-Related Proteins through Autophagy in the Primary Sertoli Cells.Viruses. 2023 May 29;15(6):1272. doi: 10.3390/v15061272. Viruses. 2023. PMID: 37376572 Free PMC article.
-
The S1 subunits of SARS-CoV-2 variants differentially trigger the IL-6 signaling pathway in human brain endothelial cells and downstream impact on microglia activation.NeuroImmune Pharm Ther. 2024 Jan 9;3(1):7-15. doi: 10.1515/nipt-2023-0024. eCollection 2024 Mar. NeuroImmune Pharm Ther. 2024. PMID: 38532784 Free PMC article.
-
Negative regulation of angiogenesis and the MAPK pathway may be a shared biological pathway between IS and epilepsy.PLoS One. 2023 Oct 4;18(10):e0286426. doi: 10.1371/journal.pone.0286426. eCollection 2023. PLoS One. 2023. PMID: 37792772 Free PMC article.
-
Protein profiling and assessment of amyloid beta levels in plasma in canine refractory epilepsy.Front Vet Sci. 2023 Dec 21;10:1258244. doi: 10.3389/fvets.2023.1258244. eCollection 2023. Front Vet Sci. 2023. PMID: 38192726 Free PMC article.
-
Towards Novel Biomimetic In Vitro Models of the Blood-Brain Barrier for Drug Permeability Evaluation.Bioengineering (Basel). 2023 May 10;10(5):572. doi: 10.3390/bioengineering10050572. Bioengineering (Basel). 2023. PMID: 37237642 Free PMC article. Review.
References
-
- Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls. 2022. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

