Ribosomal protein RPL11 haploinsufficiency causes anemia in mice via activation of the RP-MDM2-p53 pathway
- PMID: 36435197
- PMCID: PMC9793318
- DOI: 10.1016/j.jbc.2022.102739
Ribosomal protein RPL11 haploinsufficiency causes anemia in mice via activation of the RP-MDM2-p53 pathway
Abstract
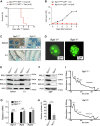
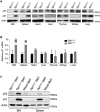
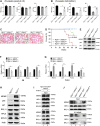
Recent discovery of the ribosomal protein (RP) RPL11 interacting with and inhibiting the E3 ubiquitin ligase function of MDM2 established the RP-MDM2-p53 signaling pathway, which is linked to biological events, including ribosomal biogenesis, nutrient availability, and metabolic homeostasis. Mutations in RPs lead to a diverse array of phenotypes known as ribosomopathies in which the role of p53 is implicated. Here, we generated conditional RPL11-deletion mice to investigate in vivo effects of impaired RP expression and its functional connection with p53. While deletion of one Rpl11 allele in germ cells results in embryonic lethality, deletion of one Rpl11 allele in adult mice does not affect viability but leads to acute anemia. Mechanistically, we found RPL11 haploinsufficiency activates p53 in hematopoietic tissues and impedes erythroid precursor differentiation, resulting in insufficient red blood cell development. We demonstrated that reducing p53 dosage by deleting one p53 allele rescues RPL11 haploinsufficiency-induced inhibition of erythropoietic precursor differentiation and restores normal red blood cell levels in mice. Furthermore, blocking the RP-MDM2-p53 pathway by introducing an RP-binding mutation in MDM2 prevents RPL11 haploinsufficiency-caused p53 activation and rescues the anemia in mice. Together, these findings demonstrate that the RP-MDM2-p53 pathway is a critical checkpoint for RP homeostasis and that p53-dependent cell cycle arrest of erythroid precursors is the molecular basis for the anemia phenotype commonly associated with RP deficiency.
Keywords: MDM2; RPL11; anemia; p53; ribosomal protein; ribosomopathy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Mice with a Mutation in the Mdm2 Gene That Interferes with MDM2/Ribosomal Protein Binding Develop a Defect in Erythropoiesis.PLoS One. 2016 Apr 4;11(4):e0152263. doi: 10.1371/journal.pone.0152263. eCollection 2016. PLoS One. 2016. PMID: 27042854 Free PMC article.
-
The RP-Mdm2-p53 pathway and tumorigenesis.Oncotarget. 2011 Mar;2(3):234-8. doi: 10.18632/oncotarget.228. Oncotarget. 2011. PMID: 21406728 Free PMC article. Review.
-
Disruption of the RP-MDM2-p53 pathway accelerates APC loss-induced colorectal tumorigenesis.Oncogene. 2017 Mar;36(10):1374-1383. doi: 10.1038/onc.2016.301. Epub 2016 Sep 12. Oncogene. 2017. PMID: 27617574 Free PMC article.
-
Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability.Oncotarget. 2014 Feb 28;5(4):860-71. doi: 10.18632/oncotarget.1784. Oncotarget. 2014. PMID: 24658219 Free PMC article. Review.
-
SRBD1 Regulates the Cell Cycle, Apoptosis, and M2 Macrophage Polarization via the RPL11-MDM2-p53 Pathway in Glioma.Environ Toxicol. 2025 Jan;40(1):66-78. doi: 10.1002/tox.24396. Epub 2024 Sep 11. Environ Toxicol. 2025. PMID: 39258423
Cited by
-
DBA syndrome 2.0: Rpl11 mouse model revisited.Blood Adv. 2025 Jun 10;9(11):2706-2708. doi: 10.1182/bloodadvances.2025016115. Blood Adv. 2025. PMID: 40440009 Free PMC article. No abstract available.
-
Silencing RPL11 attenuates acute kidney injury by suppressing tubular apoptosis and macrophage-driven inflammation.Front Immunol. 2025 Aug 15;16:1642446. doi: 10.3389/fimmu.2025.1642446. eCollection 2025. Front Immunol. 2025. PMID: 40895534 Free PMC article.
-
Interaction of Camptothecin Anticancer Drugs with Ribosomal Proteins L15 and L11: A Molecular Docking Study.Molecules. 2023 Feb 15;28(4):1828. doi: 10.3390/molecules28041828. Molecules. 2023. PMID: 36838813 Free PMC article.
-
The Central Role of Ribosomal Proteins in p53 Regulation.Cancers (Basel). 2025 May 8;17(10):1597. doi: 10.3390/cancers17101597. Cancers (Basel). 2025. PMID: 40427096 Free PMC article.
-
Features and mechanisms of long-lived Myotis somatic fibroblasts in response to DNA replication stress.Zool Res. 2025 May 18;46(3):709-721. doi: 10.24272/j.issn.2095-8137.2024.373. Zool Res. 2025. PMID: 40407135 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

