Modified exosomal SIRPα variants alleviate white matter injury after intracerebral hemorrhage via microglia/macrophages
- PMID: 36435797
- PMCID: PMC9701394
- DOI: 10.1186/s40824-022-00311-4
Modified exosomal SIRPα variants alleviate white matter injury after intracerebral hemorrhage via microglia/macrophages
Abstract
Background: Despite limited efficiency, modulation of microglia/macrophages has shown to attenuate neuroinflammation after intracerebral hemorrhage (ICH). In this context, we evaluated the efficacy of modified exosomal signal regulatory protein α (SIRPα) variants (SIRPα-v Exos) in microglia/macrophages and neuroinflammation-associated white matter injury after ICH.
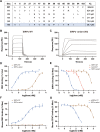
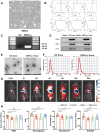
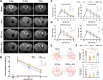
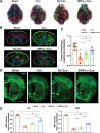
Methods: SIRPα-v Exos were engineered to block CD47-SIRPα interactions. After obtaining SIRPα-v Exos from lentivirus-infected mesenchymal stem cells, C57BL/6 mice suffering from ICH underwent consecutive intravenous injections of SIRPα-v Exos (6 mg/kg) for 14 days. Afterwards, the volume of hematoma and neurological dysfunctions were assessed in mice continuously until 35 days after ICH. In addition, demyelination, electrophysiology and neuroinflammation were evaluated. Furthermore, the mechanisms of microglial regulation by SIRPα-v Exos were investigated in vitro under coculture conditions.
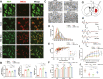
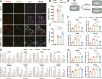
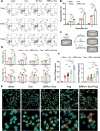
Results: The results demonstrated that the clearance of hematoma in mice suffering from ICH was accelerated after SIRPα-v Exo treatment. SIRPα-v Exos improved long-term neurological dysfunction by ameliorating white matter injury. In addition, SIRPα-v Exos recruited regulatory T cells (Tregs) to promote M2 polarization of microglia/macrophages in the peri-hematoma tissue. In vitro experiments further showed that SIRPα-v Exos regulated primary microglia in a direct and indirect manner in synergy with Tregs.
Conclusion: Our studies revealed that SIRPα-v Exos could accelerate the clearance of hematoma and ameliorate secondary white matter injury after ICH through regulation of microglia/macrophages. SIRPα-v Exos may become a promising treatment for ICH in clinical practice.
Keywords: Exosome; Intracerebral hemorrhage; Microglia/macrophages; SIRPα variant; White matter injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Recruitment of regulatory T cells with rCCL17 promotes M2 microglia/macrophage polarization through TGFβ/TGFβR/Smad2/3 pathway in a mouse model of intracerebral hemorrhage.Exp Neurol. 2023 Sep;367:114451. doi: 10.1016/j.expneurol.2023.114451. Epub 2023 May 29. Exp Neurol. 2023. PMID: 37257716
-
Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization.Drug Des Devel Ther. 2020 Aug 5;14:3143-3158. doi: 10.2147/DDDT.S255828. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32821084 Free PMC article.
-
RvE1/ChemR23 facilitates hematoma clearance and promotes M2 polarization of macrophages/microglia in intracerebral hemorrhage.Exp Neurol. 2025 Apr;386:115173. doi: 10.1016/j.expneurol.2025.115173. Epub 2025 Jan 31. Exp Neurol. 2025. PMID: 39892453
-
The Critical Role of Erythrolysis and Microglia/Macrophages in Clot Resolution After Intracerebral Hemorrhage: A Review of the Mechanisms and Potential Therapeutic Targets.Cell Mol Neurobiol. 2023 Jan;43(1):59-67. doi: 10.1007/s10571-021-01175-3. Epub 2022 Jan 4. Cell Mol Neurobiol. 2023. PMID: 34981286 Free PMC article. Review.
-
Brain injury and repair after intracerebral hemorrhage: The role of microglia and brain-infiltrating macrophages.Neurochem Int. 2021 Jan;142:104923. doi: 10.1016/j.neuint.2020.104923. Epub 2020 Nov 25. Neurochem Int. 2021. PMID: 33248206 Free PMC article. Review.
Cited by
-
Neutrophil-like pH-responsive pro-efferocytic nanoparticles improve neurological recovery by promoting erythrophagocytosis after intracerebral hemorrhage.Theranostics. 2024 Jan 1;14(1):283-303. doi: 10.7150/thno.90370. eCollection 2024. Theranostics. 2024. PMID: 38164152 Free PMC article.
-
Beyond cancer: The potential application of CD47-based therapy in non-cancer diseases.Acta Pharm Sin B. 2025 Feb;15(2):757-791. doi: 10.1016/j.apsb.2024.11.018. Epub 2024 Nov 28. Acta Pharm Sin B. 2025. PMID: 40177549 Free PMC article. Review.
-
Exosomes: A Cellular Communication Medium That Has Multiple Effects On Brain Diseases.Mol Neurobiol. 2024 Sep;61(9):6864-6892. doi: 10.1007/s12035-024-03957-4. Epub 2024 Feb 14. Mol Neurobiol. 2024. PMID: 38356095 Review.
-
White Matter Injury in Central Nervous System Disorders.Neuropsychiatr Dis Treat. 2025 Jan 24;21:107-114. doi: 10.2147/NDT.S498660. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 39877856 Free PMC article. Review.
-
From bench to bedside: nanomedicine development for intracerebral hemorrhage - exploring microenvironment, innovation, and translation.J Nanobiotechnology. 2025 Aug 14;23(1):567. doi: 10.1186/s12951-025-03661-y. J Nanobiotechnology. 2025. PMID: 40813702 Free PMC article. Review.
References
-
- Asch C, Luitse MJ, Rinkel GJ, Tweel IKlijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

