CDH17 nanobodies facilitate rapid imaging of gastric cancer and efficient delivery of immunotoxin
- PMID: 36435809
- PMCID: PMC9701387
- DOI: 10.1186/s40824-022-00312-3
CDH17 nanobodies facilitate rapid imaging of gastric cancer and efficient delivery of immunotoxin
Abstract
Background: It is highly desirable to develop new therapeutic strategies for gastric cancer given the low survival rate despite improvement in the past decades. Cadherin 17 (CDH17) is a membrane protein highly expressed in cancers of digestive system. Nanobody represents a novel antibody format for cancer targeted imaging and drug delivery. Nanobody targeting CHD17 as an imaging probe and a delivery vehicle of toxin remains to be explored for its theragnostic potential in gastric cancer.
Methods: Naïve nanobody phage library was screened against CDH17 Domain 1-3 and identified nanobodies were extensively characterized with various assays. Nanobodies labeled with imaging probe were tested in vitro and in vivo for gastric cancer detection. A CDH17 Nanobody fused with toxin PE38 was evaluated for gastric cancer inhibition in vitro and in vivo.
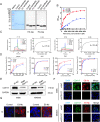
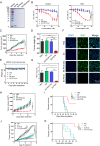
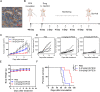
Results: Two nanobodies (A1 and E8) against human CDH17 with high affinity and high specificity were successfully obtained. These nanobodies could specifically bind to CDH17 protein and CDH17-positive gastric cancer cells. E8 nanobody as a lead was extensively determined for tumor imaging and drug delivery. It could efficiently co-localize with CDH17-positive gastric cancer cells in zebrafish embryos and rapidly visualize the tumor mass in mice within 3 h when conjugated with imaging dyes. E8 nanobody fused with toxin PE38 showed excellent anti-tumor effect and remarkably improved the mice survival in cell-derived (CDX) and patient-derived xenograft (PDX) models. The immunotoxin also enhanced the anti-tumor effect of clinical drug 5-Fluorouracil.
Conclusions: The study presents a novel imaging and drug delivery strategy by targeting CDH17. CDH17 nanobody-based immunotoxin is potentially a promising therapeutic modality for clinical translation against gastric cancer.
Keywords: Cadherin-17; Gastric cancer; Immunotoxin; Jingbo Ma, Xiaolong Xu and Chunjin Fu are contribute equally to this work and share the first-authorship.; Nanobody; Targeted therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Cadherin 17 Nanobody-Mediated Near-Infrared-II Fluorescence Imaging-Guided Surgery and Immunotoxin Delivery for Colorectal Cancer.Biomater Res. 2024 Jun 21;28:0041. doi: 10.34133/bmr.0041. eCollection 2024. Biomater Res. 2024. PMID: 38911825 Free PMC article.
-
Development and Characterization of the [177Lu]Lu-Labeled Anti-CDH17 Nanobody Derivative for Radioimmunotherapy in the Gastric Cancer Xenograft Model.Mol Pharm. 2025 Apr 7;22(4):2077-2087. doi: 10.1021/acs.molpharmaceut.4c01285. Epub 2025 Mar 15. Mol Pharm. 2025. PMID: 40088168
-
Synergistic Cytotoxic Effect on Gastric Cancer Cells of an Immunotoxin Cocktail in Which Antibodies Recognize Different Epitopes on CDH17.Monoclon Antib Immunodiagn Immunother. 2018 Feb;37(1):1-11. doi: 10.1089/mab.2017.0043. Monoclon Antib Immunodiagn Immunother. 2018. PMID: 29474162
-
Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics.Front Immunol. 2017 Nov 22;8:1603. doi: 10.3389/fimmu.2017.01603. eCollection 2017. Front Immunol. 2017. PMID: 29213270 Free PMC article. Review.
-
Nanobody Conjugates for Targeted Cancer Therapy and Imaging.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211010117. doi: 10.1177/15330338211010117. Technol Cancer Res Treat. 2021. PMID: 33929911 Free PMC article. Review.
Cited by
-
Targeted drug delivery using nanobodies to deliver effective molecules to breast cancer cells: the most attractive application of nanobodies.Cancer Cell Int. 2024 Feb 10;24(1):67. doi: 10.1186/s12935-024-03259-8. Cancer Cell Int. 2024. PMID: 38341580 Free PMC article. Review.
-
Single-domain antibodies as therapeutics for solid tumor treatment.Acta Pharm Sin B. 2024 Jul;14(7):2854-2868. doi: 10.1016/j.apsb.2024.03.016. Epub 2024 Mar 15. Acta Pharm Sin B. 2024. PMID: 39027249 Free PMC article. Review.
-
NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions.Int J Mol Sci. 2023 Aug 25;24(17):13229. doi: 10.3390/ijms241713229. Int J Mol Sci. 2023. PMID: 37686035 Free PMC article. Review.
-
Rapid diagnostic imaging and targeted immunotoxin delivery in aggressive prostate cancer using CEACAM5-specific nanobodies.J Nanobiotechnology. 2025 Jul 18;23(1):525. doi: 10.1186/s12951-025-03600-x. J Nanobiotechnology. 2025. PMID: 40682047 Free PMC article.
-
Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years.J Nanobiotechnology. 2024 Oct 26;22(1):661. doi: 10.1186/s12951-024-02900-y. J Nanobiotechnology. 2024. PMID: 39455963 Free PMC article. Review.
References
-
- Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–730. doi: 10.1038/s41586-021-04161-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

