Creb5 coordinates synovial joint formation with the genesis of articular cartilage
- PMID: 36435829
- PMCID: PMC9701237
- DOI: 10.1038/s41467-022-35010-0
Creb5 coordinates synovial joint formation with the genesis of articular cartilage
Abstract
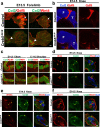
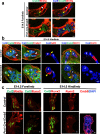
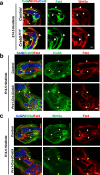
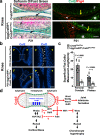
While prior work has established that articular cartilage arises from Prg4-expressing perichondrial cells, it is not clear how this process is specifically restricted to the perichondrium of synovial joints. We document that the transcription factor Creb5 is necessary to initiate the expression of signaling molecules that both direct the formation of synovial joints and guide perichondrial tissue to form articular cartilage instead of bone. Creb5 promotes the generation of articular chondrocytes from perichondrial precursors in part by inducing expression of signaling molecules that block a Wnt5a autoregulatory loop in the perichondrium. Postnatal deletion of Creb5 in the articular cartilage leads to loss of both flat superficial zone articular chondrocytes coupled with a loss of both Prg4 and Wif1 expression in the articular cartilage; and a non-cell autonomous up-regulation of Ctgf. Our findings indicate that Creb5 promotes joint formation and the subsequent development of articular chondrocytes by driving the expression of signaling molecules that both specify the joint interzone and simultaneously inhibit a Wnt5a positive-feedback loop in the perichondrium.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

